Scroll to:
Maternal blood lead level and its impact on cord blood hematological parameters
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.496
Abstract
Introduction. More than 13 annual million deaths are caused by environmental pollutants worldwide. Urbanization, population growth, industrialization and globalization affect our lives both positively and negatively. Women can become lead exposed through occupational and environmental sources. Once lead enters the body, it is mainly deposited in diverse organs: brain, kidneys, liver and bones. The body stores lead mainly in the bones, where it accumulates over time that may be further released into the bloodstream during pregnancy, thus posing a threat to growing fetus.
Aim: to examine a lead impact on newborn hematological parameters during perinatal period.
Materials and Methods. A retrospective cohort study with 306 pregnant women and paired newborns was carried out. Peripheral blood lead level (BLL) in pregnant and postpartum women was analyzed by using the atomic-absorption spectrophotometry method. Blood specimens were collected for analysis in the third trimester of pregnancy. Newborns hemoglobin concentration (mean corpuscular hemoglobin concentration, MCHC) in erythrocytes was also assessed.
Results. We have detected a statistically significant decrease of MCHC in babies born to mothers with BLL > 0.24 µmol/L vs. BLL < 0.24 µmol/L. This difference may indicate a decline in hemoglobin fetal production caused by lead intoxication.
Conclusion. Study corroborates an idea that pregnant women with occupational or environmental lead exposure should be monitored for BLL, which should not exceed 0.24 µmol/L during pregnancy.
Keywords
For citations:
Gorgadze N.T., Ungiadze J.Yu., Akhvlediani L.T., Ungiadze D.D., Baziari V.O., Lomauri Kh.N., Kokaia N.Zh., Giorgobiani M.Sh. Maternal blood lead level and its impact on cord blood hematological parameters. Obstetrics, Gynecology and Reproduction. 2024;18(2):211-217. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.496
Introduction / Введение
Lead is a widespread environmental pollutant holding the second place as most toxic metal after arsenic (As), which comprises 0.002 % of the Earth's crust. Women can become exposed to lead through occupational and environmental sources. Once lead enters the body, it is mainly deposited in diverse organs: brain, kidneys, liver and bones. The body stores lead mainly in the bones, where it accumulates over time that may be further released into the bloodstream during pregnancy, thus posing a threat to growing fetus. However, no verified safe blood lead concentration has been identified; even as low as 0.17 µmol/L blood lead level (BLL) may be associated with neurological disorders [1][2].
Lead freely crosses the placenta, resulting in its similar concentrations in maternal and newborn umbilical cord blood [3]. There is a large body of publications assessing lead exposure during pregnancy highlighted by variable presentations in newborns. Women of childbearing age chronically exposed to lead occupationally or environmentally showed high rates of sterility, fetal and neonatal demise, with paired newborns suffering from growth retardation and diverse neurologic symptoms [4].
It is known that lead is able to interfere with the activity of the hemoglobin biosynthesis enzymes such as δ-aminolevulinic acid dehydratase (ALAD), copropor-phyrinogen oxidase (COIX) and ferrochelatase (FECH), thereby affecting erythrocyte life span. Nevertheless, the lead impact on fetal hematopoietic system has not been thoroughly studied [5].
Study by О. La-Llave-Leon et al. with 292 Mexico pregnant women indicate that hemoglobin and hematocrit levels were elevated in women with BLL > 0.24 µmol/L, whereas erythrocyte mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) remained unaffected [6].
Here, we hypothesize that lead penetrates placenta to interfere with fetal erythrocyte generation. The study was carried out to investigate an impact of maternal BLL on fetal red blood cell count.
Aim: to examine a lead impact on newborn hematological parameters during perinatal period.
Materials and Methods / Материалы и методы
Study design / Дизайн исследования
Our prospective cohort study was carried out in the Autonomous Republic of Adjara, located in the southwestern part of Georgia on the Black Sea coast and Turkey borders. Batumi, the administrative center of Adjara, has 609,000 women of childbearing age (20 to 40 years old). This region which is mainly agrarian, has also an oil industry which is a source of lead pollution.
The study included prospectively enrolled 306 pregnant women and paired newborns who were admitted to the Neonatal intensive care unit (NICU) at the Batumi Medical Center LLC and Iris Borchashvili Health Center ''Medina'' for various reasons. Maternal blood was collected to analyze BLL into test tubes containing ethylenediaminetetraacetic acid (anticoagulant) under aseptic conditions, shortly after delivery. Umbilical venous blood samples were obtained in vacuum tube containing k3 EDTA anticoagulant manufactured by Greiner bio-one. Complete blood count (CBC) was measured in umbilical venous blood of neonates admitted to the NICU.
Inclusion and exclusion criteria / Критерии включения и исключения
Inclusion criteria: pregnant women who were registered at the Batumi Medical Center LLC and Iris Borchashvili Health Center ''Medina'', and whose venous blood lead concentration was assessed during pregnancy. Additionally, paired newborns, who were admitted to the NICU with various issues within the first day of life, were also included.
Еxclusion criteria: pregnant women with paired asymptomatic, healthy newborns, as well as those whose blood lead concentration was not assessed or pregnancy termination was documented despite blood lead concentration determination.
Study groups / Группы обследованных
To assess an impact of maternal BLL on newborn red blood cell production, subjects were divided into 2 groups based on maternal BLL: group 1 – maternal BLL < 0.24 μmol/L (n = 192), and group 2 – maternal BLL ≥ 0.24 μmol/L (n = 114). A cut-off BLL was set at 0.24 μmol/L since it is considered toxic in humans. Groups were compared by birth weight (g), gestational age (GA, weeks), red blood cells count (RBC, ×10¹²/L), hemoglobin level (Hb, g/L), mean corpuscular volume (MCV, fl), mean corpuscular hemoglobin concentration (MCHC, g/L) and mean corpuscular hemoglobin (MCH, pg).
Blood samples were collected from newborns within the first 6 hours of life. Sampling was conducted from the umbilical vein using a vacuum tube containing K3 EDTA anticoagulant manufactured by Greiner Bio-One. The analysis was performed using an XT-4000i hematological analyzer that undergoes regular annual manual engineering maintenance and daily internal quality control checks.
Blood lead level determination / Определение уровня свинца в крови
Maternal BLL was measured using atomic absorption spectrophotometry (Zeeman system model: AA240 Zeeman, Agilent Technologies, USA), calibrated with lead standard reference solution (1000 μg/mL stock Lead Solution (Pb), Lot. No 782761), intermediate standards and two control samples (spikes) prepared for quality assurance.
Ethical aspects / Этические аспекты
Written informed consents were obtained from the patients’ legal guardian/close relative for publication of any potentially identifiable images or data included in the article. The study was conducted based on the approval of the Ethics Committee of the healthcare center "Medina", protocol No. 01-1405/21 and the study was conducted according to the ethical standards of the Helsinki Declaration of the 1964 World Medical Association and its subsequent updates.
The patient's legal guardian or close relative provided written informed consent for publication of any data included in the article.
Statistical methods / Статистические методы
Statistical analysis was performed using NCSS software (NCSS LLC., USA). Categorical variables were described as frequency and percentage. Continuous variables were evaluated for normal distribution using histogram and Kolmogorov–Smirnov test and were reported as mean ± standard deviation (M ± SD) or median and interquartile range (Me [ Q25; Q75]). Independent samples were analyzed using t-test and Mann-Whitney test to compare continuous inter-group variables and χ² test was applied to assess an association birth before week 38. All statistical assays were two sided, with p < 0.05 considered as statistically significant.
Results / Результаты
In 306 pregnant women, whose newborns were admitted to the NICU at Iris Borchashvili Health Center ''Medina'', the average BLL was 0,25 μmol/L with a standard deviation of 0.192 (range: 0.012–1,368) μmol/L.
192 (62.75 %) and 114 (32.25 %) mothers enrolled in the study had BLL < 0.24 μmol/L and BLL of ≥ 0.24 μmol/L, respectively; 60 (19.6 %) and 246 (80.4 %) paired babies were born at gestational age < 38 and ≥ 38 weeks, respectively (Table 1, 2).
Table 1. Maternal blood lead level.
Таблица 1. Уровень свинца в материнской крови.
|
Group / Группа |
n |
Mean ± SD |
Me [ Q25; Q75] |
|
Maternal blood lead level < 0.24 μmol/L Уровень свинца в материнской крови < 0,24 мкмоль/л |
192 |
0.23 ± 0.04 |
0.144 [ 0.106; 0,187] |
|
Maternal blood lead level ≥ 0.24 μmol/L Уровень свинца в материнской крови ≥ 0,24 мкмоль/л |
114 |
0.432 ± 0.202 |
0.379 [ 0.300; 0.485] |
Table 2. Demographic and hematological parameters.
Таблица 2. Демографические и гематологические параметры.
|
Parameter Показатель |
Maternal blood lead level Уровень свинца в материнской крови |
p |
|
|
< 0.24 μmol/L < 0,24 мкмоль/л n = 192 |
≥ 0.24 μmol/L ≥ 0,24 мкмоль/л n = 114 |
||
|
Gestational age, weeks Гестационный возраст, недель Mean ± SD Me [ Q25; Q75] |
34.8 ± 3.6 35.1 [ 32.6; 37.3] |
35.3 ± 3.3 36.0 [ 34.4; 37.3] |
N/S |
|
Birth weight, g Масса тела при рождении, г Mean ± SD Me [ Q25; Q75] |
2338 ± 818 2325 [ 1800; 2950] |
2378 ± 828 2400 [ 1800; 3000] |
N/S |
|
Red blood cell count, ×10¹²/L Эритроциты, ×10¹²/L Mean ± SD Me [ Q25; Q75] |
4.67 ± 0.83 4.68 [ 4.22; 5.18] |
4.69 ± 0.85 4.72 [ 4.30; 5.30] |
N/S |
|
Hemoglobin, g/L Гемоглобин, г/л Mean ± SD Me [ Q25; Q75] |
170.1 ± 28.3 168 [ 155; 187] |
170.4 ± 31.0 172 [ 157; 191] |
N/S |
|
Mean corpuscular hemoglobin concentration, g/L Средняя концентрация гемоглобина в эритроците, г/л Mean ± SD Me [ Q25; Q75] |
347.5 ± 20.4 348 [ 340.2; 357] |
343.2 ± 14.1 342 [ 337.7; 349] |
< 0.05 |
|
Mean corpuscular volume, fl Средний объем эритроцитов, фл Mean ± SD Me [ Q25; Q75] |
105.1 ± 7.3 104.5 [ 100.6; 108.7] |
106.1 ± 7.7 105.4 [ 102.1; 109.6] |
N/S |
|
Mean corpuscular hemoglobin, pg Среднее содержание гемоглобина в эритроците, пг Mean ± SD Me [ Q25; Q75] |
36.5 ± 2.2 36.3 [ 35.1; 37.8] |
36.1 ± 3.6 36.1 [ 35.8; 37.9] |
N/S |
Note: N/S – non-significant.
Примечание: N/S – различия незначимы.
A significant inter-group difference (p < 0.05) in MCHC level is shown in Table 2 and box-plot (Fig. 1).
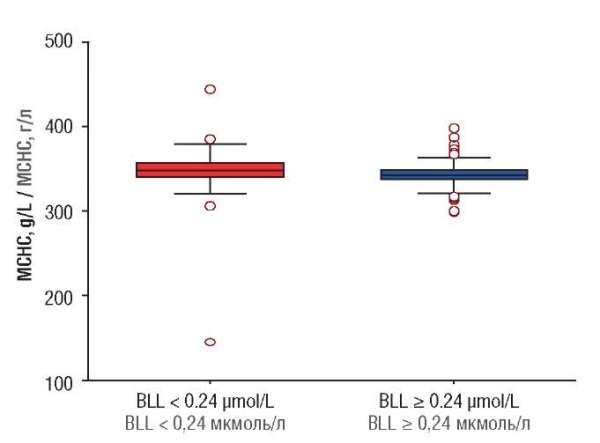
Figure 1. Mean corpuscular hemoglobin concentration (MCHC).
Note: BLL – blood lead level.
Рисунок 1. Cредняя концентрация гемоглобина в эритроците (MCHC).
Примечание: BLL – уровень свинца в крови.
Discussion / Обсуждение
Urbanization, population growth, industrialization and globalization affect human life both positively and negatively. Despite the benefits from the economic and political perspectives, the globalization negatively impacts environment. Lead is a widespread environmental pollutant. Even low blood lead levels have been observed to be associated with increased risk of minor birth defects. The short- and long-term effects of increased prenatal lead exposure in infants remain largely unknown, except for some evidence suggesting neurodevelopmental impairment in children [6]. Experts of the World Health Organization (WHO) emphasize that lead is especially dangerous for young children due to intensive brain growth and development as well as increased nutrient demand. Lead is a cumulative metal deposited in bones, which mobilization becomes elevated during pregnancy specifically promoted in case a mother intakes no calcium supplement despite increased demand for it. Lead concentration in umbilical cord blood comprises 85 % of its level in paired maternal blood. Therefore, lead is primarily dangerous for developing fetus [7]. Our study hypothesis was that lead crossing the placenta may interfere with fetal potential for red blood cell generation.
We have detected a statistically significantly decreased hemoglobin level in erythrocyte mass (MCHC) in babies born to mothers with BLL ≥ 0.24 μmol/L vs. BLL < 0.24 μmol/L. Such finding may point at lowered hemoglobin fetal production caused by lead intoxication.
A potential mechanism account for decreased MCHC in babies born to mothers with BBL ≥ 0.24 μmol/L may result from an impact of prenatal lead on placental function. Altered placental function interferes with its vital role in regulating nutrient transport such as iron and vitamins necessary for fetal red blood cell generation.
Conclusion / Заключение
Our study cohort consisted of pregnant women whose paired newborns were admitted to the NICU. It was found that mean BLL was high reaching 0.25 μmol/L that exceeds that of set by the the Centers for Disease Control and Prevention (CDC) for pregnant women. There were examined cord blood samples from babies born to study women showing that MCHC was lower in newborns at maternal BLL ≥ 0.24 μmol/L.
Our study corroborates an idea that pregnant women with occupational or environmental lead exposure should be monitored for BLL, which should not exceed 0.24 µmol/L during pregnancy.
A mechanism underlying lead effects interfering with hemoglobin fetal production requires to be further investigated.
References
1. Pacer E.J., Palmer C.D., Parsons P.J. Determination of lead in blood by graphite furnace atomic absorption spectrometry with Zeeman background correction: Improving a well-established method to support a lower blood lead reference value for children. Spectrochimica Acta Part B-Atomic Spectroscopy. 2022;190:106324. doi: 10.1016/j.sab.2021.106324.
2. US CDC Advisory Committee on Childhood Lead Poisoning Prevention. CDC updates blood lead reference value to 3.5 µg/dL. Atlanta: US Centers for Disease Control and Prevention, 2021. Available at: https://www.cdc.gov/lead-prevention/php/news-features/updates-blood-lead-reference-value.html?CDC_AAref_Val=https://www.cdc.gov/nceh/lead/news/cdc-updates-blood-lead-reference-value.html. [Accessed: 15. 01. 2024].
3. Ryu J.E., Ziegler E.E., Fomon S.J. Maternal lead exposure and blood lead concentration in infancy. J Pediatr. 1978;93(3):476–8. doi: 10.1016/s0022-3476(78)81169-x.
4. Angle C.R., McIntire M.S. Lead poisoning during pregnancy. Fetal tolerance of calcium disodium edetate. Am J Dis Child. 1964;108:436–9. doi: 10.1001/archpedi.1964.02090010438016.
5. Arshad S., Arif A., Wattoo J.I. Response of iron deficiency markers to blood lead levels and synergistic outcomes at prenatal stage. Dose Response. 2022;20(2):15593258221101744. doi: 10.1177/15593258221101744.
6. La-Llave-Leon O., Lugo-Soto R., Aguilar-Duran M. et al. Relationship between blood lead levels and hematological indices in pregnant women. Women Health. 2015;55(1):90–102. doi: 10.1080/03630242.2014.972019.
7. Charkiewicz A.E., Backstrand J.R. Lead toxicity and pollution in Poland. Int J Environ Res Public Health. 2020;17(12):4385. doi: 10.3390/ijerph17124385.
About the Authors
N. T. GorgadzeGeorgia
Nato T. Gorgadze, MD, Postgraduate Student, Neonatologist, Head of Department
Department of Neonatology
25 Ilia Chavchavadze Avenue, Tbilisi 0179; 237 Fridon Khalvashi Avenue, Batumi, Autonomous Republic of Adjara 6010
Ju. Yu. Ungiadze
Georgia
Jumber Yu. Ungiadze, MD, Dr Sci Med, Director, Professor
Faculty of Medicine
6010; 35 Ninoshvili Str.; 237 Fridon Khalvashi Avenue; Autonomous Republic of Adjara; Batumi
L. T. Akhvlediani
Georgia
Leila T. Akhvlediani, MD, PhD (Biology), Associate Professor, Professor of Immunology, Dean
Medical Faculty
6010; 35 Ninoshvili Str.; 237 Fridon Khalvashi Avenue; Autonomous Republic of Adjara; Batumi
Researcher ID: AAD-4983-2021
D. D. Ungiadze
Georgia
Davit D. Ungiadze, Lecturer, Clinical Resident
Department of Obstetrics and Gynecology
6010; 237 Fridon Khalvashi Avenue; Autonomous Republic of Adjara; Batumi; 0144; 51/2 Ketevan Tsamebuli Avenue; Tbilisi
V. O. Baziari
Georgia
Vera O. Baziari, MD, Dr Sci Med
0186; 33 Vazha Pshavela Avenue; Tbilisi
Researcher ID: KDP-0325-2024
Kh. N. Lomauri
Georgia
Khatuna N. Lomauri, MD, Dr Sci Med, Professor, Head of Department
Neonatology Department
0186; 33 Vazha Pshavela Avenue; Tbilisi
N. Zh. Kokaia
Georgia
M.Nora Zh. Kokaia, MD, Dr Sci Med, Professor
0144; 51/2 Ketevan Tsamebuli Avenue; Tbilisi
M. Sh. Giorgobiani
Georgia
Manana Sh. Giorgobiani, MD, Dr Sci Med, Professor
0179; 25 Ilia Chavchavadze Avenue; Tbilisi
What is already known about this subject?
► Lead is a widespread environmental pollutant holding the second place as most toxic metal after arsenic, which comprises 0.002 % of the Earth's crust.
► There is a large body of literature assessing lead exposure during pregnancy highlighted by variable presentations in newborns.
► Women of childbearing age chronically exposed to lead occupationally or environmentally have shown high rates of sterility, fetal and neonatal demise, with paired newborns suffering from growth retardation and diverse neurologic symptoms.
What are the new findings?
► A statistically significantly decreased mean corpuscular hemoglobin concentration (MCHC) in babies born to mothers with blood lead level (ВLL) > 0.24 μmol/L vs. BLL < 0.24 μmol/L was detected that may evidence about lowered hemoglobin fetal production caused by lead intoxication.
How might it impact on clinical practice in the foreseeable future?
► Study corroborates an idea that pregnant women with occupational or environmental lead exposure should be monitored for BLL, which must be less 0.24 µmol/L during pregnancy.
Review
For citations:
Gorgadze N.T., Ungiadze J.Yu., Akhvlediani L.T., Ungiadze D.D., Baziari V.O., Lomauri Kh.N., Kokaia N.Zh., Giorgobiani M.Sh. Maternal blood lead level and its impact on cord blood hematological parameters. Obstetrics, Gynecology and Reproduction. 2024;18(2):211-217. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.496

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































