Scroll to:
Clinical significance of assessing ADAMTS-13 and von Willebrand factor level in COVID-19 convalescent pregnant women
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.386
Abstract
Introduction. Coronavirus infection is associated with severe endotheliopathy, thromboinflammation and immunothrombosis leading to excessive release of von Willebrand factor (vWF) multimers from Weibel–Palade bodies, which can affect activity of ADAMTS-13 metalloproteinase (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) and the ADAMTS-13/vWF axis previously shown by us to be altered in non-pregnant women with severe COVID-19.
Aim: to study a clinical role of hemostasis activation particularly ADAMTS-13/vWF axis in pregnant women after COVID-19.
Materials and Methods. A prospective case–control study was conducted with pregnant women (n = 135) divided into 3 groups: group 1 included 45 women with prior COVID-19 during pregnancy, group 2 – 45 women in the acute phase of the infection during pregnancy, group 3 – 45 healthy pregnant women. The level of vWF and ADAMTS-13 was assessed in all patients.
Results. The concentration of vWF antigen (vWF:Ag) in the acute period of the disease in pregnant women with COVID-19 was significantly higher compared to the control group (p < 0.001). ADAMTS-13 level in pregnant women after COVID-19 did not differ from that of in control group, while vWF level was significantly higher in 66.7 % (30/45). The ADAMTS-13/vWF ratio was increased and significantly differed both in pregnant patients during the acute period of the disease (p < 0.001) and pregnant women after infection (p = 0.0002) compared with the control group.
Conclusion. Our results show that endotheliopathy was prominently manifested in pregnant women with COVID-19 and persisted for several months after disease. The ADAMTS-13/vWF ratio determines the pathway functioning, the risk of microcirculation disorders and clinical complications.
For citations:
Gashimova N.R., Grigoreva K.N., Bitsadze V.O., Pankratyeva L.L., Khizroeva J.Kh., Tretyakova M.V., Shammut Ya.M., Iupatov E.I., Tsibizova V.I., Gris J., Blinov D.V., Makatsariya A.D. Clinical significance of assessing ADAMTS-13 and von Willebrand factor level in COVID-19 convalescent pregnant women. Obstetrics, Gynecology and Reproduction. 2023;17(1):8-17. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.386
Introduction / Введение
The coronavirus disease 2019 (COVID-19) remains to be a global healthcare emergency. Although the disease was initially thought to be limited to the respiratory tract, soon it became clear that it was presented as a multisystem disease causing coagulopathy, kidney fai- lure, liver dysfunction, and heart failure [1]. The severe course of the disease is more common in the elderly and in comorbidity with chronic diseases such as diabetes mellitus, hypertension, chronic obstructive pulmonary disease, coronary heart disease, and chronic kidney disease [2]. SARS-CoV-2 infection is associated with abundant thrombotic complications, such as deep vein thrombosis and pulmonary embolism [3]. As a rule, a severe course of SARS-CoV-2 infection is observed upon strong inflammatory reaction due to neutrophil release and infiltration in various organs coupled to neutrophil extracellular traps (NETs), increased plasma levels of pro-inflammatory cytokines and chemokines, leading to cytokine storm, massive damage to the endothelium as well as activation of macrophages, platelets, and endothelial cells [4][5]. One of the leading causes of death in COVID-19-patients is thrombo-inflammatory concomitant diseases, such as hypercoagulability, thrombosis, and respiratory failure due to pulmonary microvascular thrombosis [6][7].
A spectrum of clinical manifestations widely varies from asymptomatic to severe in pregnant women with COVID-19 presented from mild, common cold-like to severe symptoms with thrombotic complications [8]. It is known that SARS-CoV-2 infection during pregnancy can be an additional trigger for severe thrombotic complications [9] because pregnancy serves as a preexisting physiological hypercoagulable state. Pathophysiological phenomena underlying the increased risk of obstetric complications are mainly presented by cytokine storm and activation of circulating cells such as macrophages, T-lymphocytes, and endothelial cells. SARS-CoV-2 promotes the development of endotheliitis in various organs and tissues, leading to endothelial damage [10] and, in turn, to thrombotic microangiopathy (TMA), which is related to direst consequences of infection [11]. An important etiological factor leading to TMA is the ADAMTS-13 metalloproteinase (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) deficiency. ADAMTS-13 belongs to the family of peptidase enzymes whose biological function is to cleave von Willebrand factor (vWF) multimers. Endothelial damage results in excessively released vWF multimers from Weibel–Palade bodies followed by ADAMTS-13 consumption. It is also important to remember that NETs can inhibit ADAMTS-13 activity followed by increased vWF concentration. In turn, excessive accumulation of ultrahigh molecular weight vWF multimers in complex with platelets can cause thrombosis of the microvasculature [12]. Many publications appear in global medical literature on potential role for a relationship between ADAMTS-13 and vWF in assessing acute conditions caused by COVID-19. Usually, the balance between ADAMTS-13 and vWF plays a crucial role in maintaining average circulation in vital organs. These data suggest that acute endothelial cell activation and dysregulation of the normal ADAMTS-13/vWF axis play an essential role in the pathogenesis of underlying COVID-19 immunothrombosis. Our previous study showed that increased vWF:Ag level, decreased concentration of ADAMTS-13 as well as an imbalance in the ADAMTS-13/vWF axis significantly correlate with low survival in severe coronavirus infection [13].
A recent study by H. Fogarty et al. demonstrated that the blood plasma levels of vWF:Ag and factor VIII (FVIII) remain markedly elevated 3 months later in convalescent patients after coronavirus disease compared with healthy control group [14]. Based on these data, a role for sustained endothelial cell activation in post-COVID-19 patients can be suggested. In this study, we set a goal to study clinically significant changes in the ADAMTS-13/vWF axis in pregnant women with previous coronavirus infection.
Aim: to study a clinical role of hemostasis activation particularly ADAMTS-13/vWF axis in pregnant women after COVID-19.
Materials and Мethods / Материалы и методы
Study design / Дизайн исследования
A prospective randomized case-control study involving 135 pregnant women was conducted at the following clinical sites: Maternity hospital No. 4 at Vorokhobov City Clinical Hospital No. 67, Perinatal Center at Vorokhobov City Clinical Hospital No. 67, Agafonov Republican Clini- cal Infectious Diseases Hospital (Republic of Tatarstan).
Patient groups / Группы обследованных
The patients were divided into 3 groups: group 1 – 45 patients with COVID-19 during pregnancy, group 2 included 45 pregnant women with acute COVID-19, control group – 45 healthy pregnant women.
Inclusion and exclusion criteria / Критерии включения и исключения
Inclusion criteria: age over 18; pregnant women diagnosed with COVID-19 during pregnancy (SARS-CoV-2 positive PCR test); singleton pregnancy; voluntary informed consent to participate in the study.
Inclusion criteria for the control group: age over 18; singleton pregnancy; physiological course of pregnancy; voluntary informed consent to participate in the study.
Exclusion criteria: age under 18 years; verified active infectious and inflammatory process; confirmed positive test for antibodies to HIV; markers of viral hepatitis, syphilis; previous ARVI, vaccinated; signs of thrombotic or hemorrhagic syndrome at first examination; refusal to participate in the study.
Study methods / Методы исследования
The patient's peripheral blood samples obtained on the day of hospitalization before treatment were placed in a 3.2 % buffered sodium citrate solution and centrifuged for 20 min at 3000 g, room temperature. Plasma samples were collected and stored at –80 °С. The level of von Willebrand factor antigen (vWF:Ag), ADAMTS-13 antigen (ADAMTS-13:Ag), ADAMTS-13 activity (ADAMTS-13:Aс), ADAMTS-13 inhibitor (ADAMTS-13:i) was assessed by using commercial TECHNOZYM® test kits (Techno-clone Herstellung von Diagnostika und Arzneimitteln Gmb, Austria). According to the manufacturer, the normal reference ranges were: for ADAMTS-13:Ag – 0.41–1.41 U/mL, for ADAMTS-13:Ac – 0.4–1.3 IU/mL, for ADAMTS-13:i – less than 15 U/ml; for vWF:Ag – 0.5–1.5 IU/ml (50–150 %).
Ethical aspects / Этические аспекты
The study was approved by the Local Ethics Committee at the Sechenov University (Protocol No 04-22, dated of February 16, 2022). All patients participating in the study were informed about the scope of the study and the inclusion of examination data in current study. All patients received written informed consent.
The study was conducted following the ethical standards of the Declaration of Helsinki of the World Medical Association.
Statistical analysis / Статистический анализ
The data obtained were systematized in Microsoft Office Excel 2021 spreadsheets (Microsoft, USA). Statistical data processing was performed using the Jamovi program, version 1.2.5 (The jamovi project, Australia). Statistical analysis included the calculation of descriptive statistics: mean (M), median (Me), standard deviation (SD), and border of the 95 % confidence interval (95 % CI). While comparing quantitative data, the Mann–Whitney test was used. To test the statistical significance, a one-way analysis of variance was used by calculating Fisher's exact test at level less than 0.05 indicating significant differences. The Fisher's "p" value more than 0.05 indicated no differences.
Results and Discussion / Результаты и обсуждение
Clinical and anamnestic data as well as perinatal outcomes of the examined subjects are presented in Table 1.
The groups showed no significant difference in maternal characteristics. Two patients from group 1 and three from group 2 had arterial hypertension; in each group, 2 patients had mild preeclampsia during pregnancy. In case of COVID-19, none of the patients had signs of placental insufficiency and fetal growth retardation. In group 1, the majority of pregnant women had a mild COVID-19 (34/45; 75.5 %), 7/45 – (15.6 %) moderate form, and only 4/45 (8.9 %) – severe form, which did not significantly differ from group 2. Patients with moderate and severe COVID-19 were hospitalized for observation at infectious disease hospital. Severe cases of COVID-19 were not critical. In acute infection, 17 (37.7 %) patients from group 1 and 12 (26.6 %) from group 2 received low molecular weight heparin. No perinatal death was recorded in groups; 13.3 % (6/45) of patients in group 1, 46.6 % (21/45) of group 2, and 15.6 % (7/45) of group 3 had a history of pregnancy loss. It was found that preterm births accounted for 6.7 % (3/45) of observations in group 2. Vaginal delivery occurred in most women (91.1 %; 41/45) in either group (60.0 %; 27/45). Preterm births were observed only in group 2, accounting for 24.4 % (11/45).
Table 2 presents laboratory parameters of the pregnant women examined in the study.
Table 1. Clinical and anamnestic characteristics and perinatal outcomes.
Таблица 1. Клинико-анамнестическая характеристика и перинатальные исходы.
|
Parameter Показатель |
Group 1 Группа 1 n = 45 |
Group 2 Группа 2 n = 45 |
Control group Контрольная группа n = 45 |
|
Age, years, M Возраст, лет, M Min–max |
28,0 19–40 |
27,7 18–38 |
27,0 19–38 |
|
Body mass index before pregnancy, kg/m2, M Индекс массы тела до беременности, кг/м2, М |
25,3 |
25,8 |
24,5 |
|
Blood group, n (%): Группа крови, n (%): • blood group 0 / группа крови 0 • blood group А / группа крови А • blood group В / группа крови В • blood group АВ / группа крови АВ |
14 (31,2) 19 (42,2) 10 (22,2) 2 (4,4) |
14 (31,2) 20 (44,4) 9 (20,0) 2 (4,4) |
13 (28,9) 17 (37,8) 11 (24,4) 4 (8,9) |
|
Previous pregnancies, n (%): Предыдущие беременности, n (%): • nullipara / нерожавшие • term delivery / своевременные роды • preterm delivery / преждевременные роды • pregnancy losses / потери беременности |
28 (62,2) 25 (55,6) 0 7 (15,6) |
20 (44,4) 34 (75,5) 3 (6,7) 21 (46,7) |
23 (51,1) 29 (64,4) 0 6 (13,3) |
|
Arterial hypertension, n (%) Артериальная гипертензия, n (%) |
2 (4,4) |
3 (6,7) |
0 |
|
Preeclampsia, n (%) Преэклампсия, n (%) |
2 (4,4) |
2 (4,4) |
0 |
|
Perinatal outcomes, n (%): Перинатальные исходы, n (%): • premature rupture of membranes / преждевременное излитие околоплодных вод • preterm delivery / преждевременные роды • premature detachment of normally located placenta / преждевременная отслойка нормально расположенной плаценты • natural delivery / роды через естественные родовые пути • caesarean section delivery / роды путем операции кесарева сечения • Apgar score of less than 7 at 5 minutes / оценка по шкале Апгар на 5-й минуте менее 7 баллов |
8 (17,8) 0 0 41 (91,1) 4 (8,9) 0 |
9 (20,0) 11 (24,4) 2 (4,4) 27 (60,0) 18 (40,0) 3 (6,7) |
7 (15,6) 0 0 44 (97,8) 1 (2,2) 0 |
|
COVID-19 severity, n (%): Степень тяжести COVID-19, n (%): • mild / легкая • moderate / средняя • severe / тяжёлая |
34 (75,5) 7 (15,6) 4 (8,9) |
37 (82,2) 6 (13,3) 2 (4,4) |
0 0 0 |
|
Pregnancy trimester (+ COVID-19), n (%): Триместр беременности (+ COVID-19), n (%): • I trimester / I триместр • II trimester / II триместр • III trimester / III триместр |
6 (13,3) 19 (42,2) 20 (44,4) |
5 (11,2) 11 (24,4) 29 (64,4) |
0 0 0 |
Table 2. Laboratory parameter assessment.
Таблица 2. Результаты изученных лабораторных показателей.
|
Parameter Показатель |
Group 1 Группа 1 n = 45 |
Group 2 Группа 2 n = 45 |
Control group Контрольная группа n = 45 |
p |
|
vWF:Ag, IU/ml vWF:Ag, МЕ/мл |
2,38 ± 1,18 |
2,43 ± 0,586 |
1,382 ± 0,465 |
< 0,001* < 0,001** |
|
ADAMTS-13:Aс, IU/ml ADAMTS-13:Aс, МЕ/мл |
0,692 ± 0,188 |
0,541 ± 0,171 |
0,588 ± 0,251 |
0,029* 0,478** |
|
ADAMTS-13:Ag, U/ml ADAMTS-13:Ag, ЕД/мл |
0,843 ± 0,319 |
0,378 ± 0,149 |
0,807 ± 0,159 |
0,550* < 0,001** |
|
ADAMTS-13:Ac/vWF:Ag |
0,352 ± 0,194 |
0,237 ± 0,097 |
0,53 ± 0,458 |
0,031* < 0,001** |
|
ADAMTS-13:Ag/vWF:Ag |
0,437 ± 0,268 |
0,163 ± 0,072 |
0,708 ± 0,507 |
< 0,001* < 0,001** |
|
vWF:Ag/ADAMTS-13:Ag |
3,8676 ± 4,505 |
7,472 ± 3,638 |
1,794 ± 0,784 |
0,0002* < 0,001** |
Note: *significance of differences between group 1 and control group; **significance of differences between group 2 and control group.
Примечание: *значимость различий между группой 1 и контрольной группой; **значимость различий между группой 2 и контрольной группой.
The examined women showed significant differences in the level of vWF:Ag in acute COVID-19 (group 2) vs. control group (p < 0.001) as well as between pregnant women in the post-COVID (group 1) compared to control group (p < 0.001). There were noticeable interindividual differences in vWF:Ag level, which varied from 1.063 to 6.074 IU/ml in convalescents. Accordingly, plasma levels of vWF:Ag above the upper limit in our local reference range (0.5–1.5 IU/ml) were observed in 66.7 % (30/45) of patients with prior COVID-19 (group 1), whereas in this group it was 2.38 IU/ml being significantly higher compared to control group. In the latter, it was higher only in 40.0 % (18/45) of pregnant women, with an average concentrartion of 1.382 IU/ml (Fig. 1). Thus, a high blood plasma level of vWF:Ag in pregnant women after a coronavirus infection indicates ongoing endotheliopathy and continuing endothelial cell activation.
There were no significant differences in assessing ADAMTS-13:Ac among patients with a history of coronavirus infection (group 1) and control group as well as among patients with acute COVID-19 (group 2) vs. control group.
While assessing ADAMTS-13:i concentration, significant differences were observed between group 1 and group 2 vs. control group (Fig. 2): in healthy pregnant women (group 3) ADAMTS-13:i level was 3.144 ± 2.657 U/ml, which is profoundly lower than in group 2 – 7.3920 ± 5.817 U/ml (p < 0.001) and in group 1 – 5.619 ± 3.227 U/ml (p = 0.0002).
Significant differences were found in ADAMTS-13:Ag magnitude between pregnant women in acute COVID-19 (group 2) and healthy pregnant women (p < 0.001); no significant difference was found while comparing post-COVID (group 1) and healthy pregnant women (Fig. 3).
More than half (55.6 %; 25/45) of pregnant women during acute COVID-19 (group 2) experienced an increase in vWF:Ag concentration with simultaneously decreased ADAMTS-13 levels, which most likely resulted from the secondary consumption of ADAMTS-13 metal- loproteinase. Moreover, the vWF:Ag/ADAMTS-13:Ag ratio is significantly higher than in healthy patients (p < 0.001), demonstrating a disturbed vWF/ADAMTS-13 axis and regulation system of normal microcirculation.
In the post-COVID state (group 1), only in 6.7 % (3/45) cases was an increase in vWF:Ag magnitude with simultaneously decreased ADAMTS-13 level. Therefore, ADAMTS-13 is not consumed in such quantities as in acute COVID-19. Thus, the regulatory function of the vWF:Ag/ADAMTS-13:Ag axis remains intact in a significantly high proportion of patients (Fig. 4).
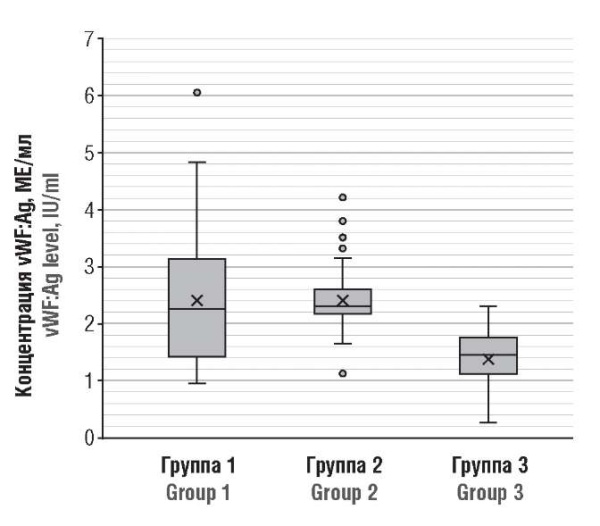
Figure 1. von Willebrand factor antigen (vWF:Ag) level in post-COVID-19 pregnant patients (Group 1), acute COVID-19 (Group 2), and healthy pregnant women (Group 3).
Рисунок 1. Концентрация антигена фактора фон Виллебранда (vWF:Ag) у беременных пациенток после перенесенного COVID-19 (группа 1), в острый период COVID-19 (группа 2) и у здоровых беременных (группа 3).
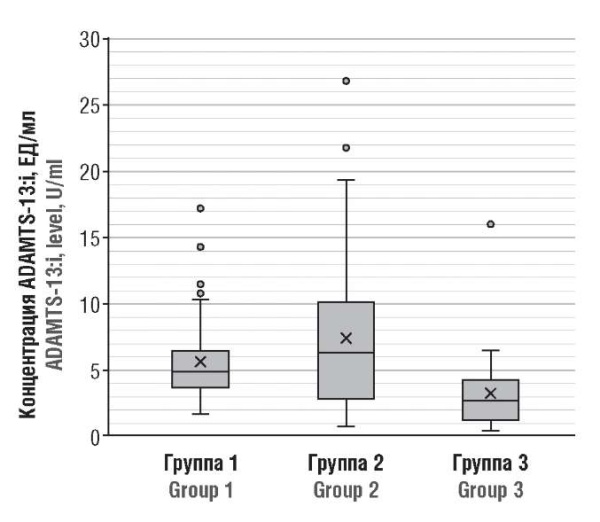
Figure 2. ADAMTS-13 inhibitor (ADAMTS-13:i) level in post-COVID-19 pregnant patients (Group 1), period COVID-19 (Group 2), and healthy pregnant women (Group 3).
Рисунок 2. Концентрация ингибитора ADAMTS-13 (ADAMTS-13:i) у беременных пациенток после коронавирусной инфекции (группа 1), в острый период болезни (группа 2) и здоровых беременных (группа 3).
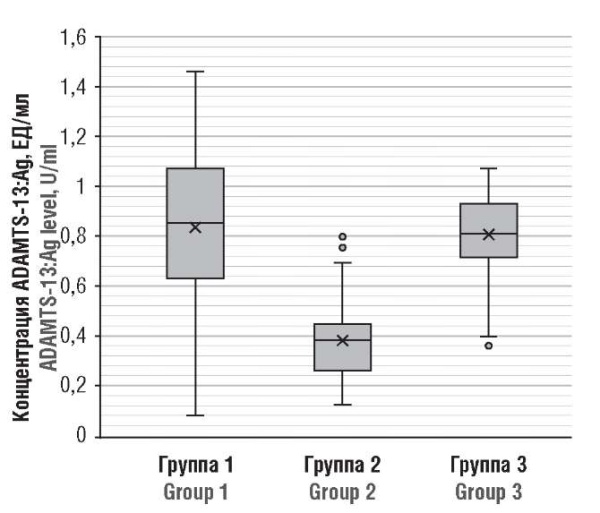
Figure 3. ADAMTS-13 antigen (ADAMTS-13:Ag) level in post-COVID-19 pregnant patients (Group 1), acute COVID-19 (Group 2), and healthy pregnant women (Group 3).
Рисунок 3. Концентрация антигена ADAMTS-13 (ADAMTS-13:Ag) у беременных пациенток после перенесенного COVID-19 (группа 1), в острый период COVID-19 (группа 2) и у здоровых беременных (группа 3).
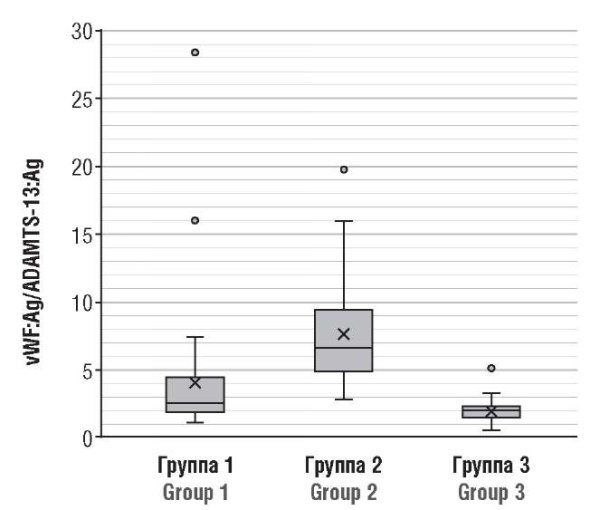
Figure 4. vWF:Ag/ADAMTS-13:Ag axis in post-COVID-19 pregnant patients (Group 1), acute COVID-19 (Group 2), and healthy pregnant women (Group 3).
Рисунок 4. Ось vWF:Ag/ADAMTS-13:Ag у беременных пациенток после перенесенного COVID-19 (группа 1), в острый период COVID-19 (группа 2) и у здоровых беременных (группа 3).
In the acute phase of COVID-19, the disease is hyper- inflammatory, prothrombotic and therefore negatively affect the level of specific thrombo-inflammatory markers, leading to endotheliopathy and increasing susceptibility to thrombotic and microvascular disorders (including microthrombosis and TMA) [15]. Therefore, it is not surprising that the ADAMTS-13/vWF axis may be involved in the TMA and cytokine storm observed during COVID-19 outside pregnancy. In non-pregnant adults, the more the ADAMTS-13/vWF axis is impaired, the more severe the disease course [13]. In this study, most pregnant women with SARS-CoV-2 infection had mild disease. However, regarding fetal and maternal outcomes, we found a significantly higher prevalence of preterm birth during acute COVID-19 (24.4 %). Noteworthy, as E. Grandone et al. pointed out that one of the most critical factors of preterm labor in women with COVID-19 is endotheliopathy, and an imbalance of vWF and ADAMTS-13 can contri- bute to multiorgan thrombosis with clinical TMA picture [16]. We consider extremely important to further elucidate these issues to confirm a role of endotheliopathy and other latent hemostasis disorders in emerging pregnancy complications.
Conclusion / Заключение
Our data provide new insight into the nature of persistent endotheliopathy and imbalance in the ADAMTS-13/vWF axis in post-COVID-19 pregnant women. Functioning of the latter is determined by assessing ADAMTS-13/vWF ratio that also accounts for risk of microcirculatory disorders as well as clinical complications. Thus, determining the ADAMTS-13/vWF ratio is crucial in clinical practice. Consistent with the critical role of immunothrombosis in acute COVID-19, our results support the hypothesis that persistent endotheliopathy and hemostatic dysfunction are sustained after COVID-19 during pregnancy, which is clinically relevant for the management of such patients. Further studies on larger patient cohort with longer follow-ups are required to confirm our data to open up new possibilities in treating post-COVID conditions.
References
1. Huang C., Wang Y., Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497– 506. https://doi.org/10.1016/S0140-6736(20)30183-5
2. Wang D., Hu B., Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. https://doi.org/10.1001/jama.2020.1585.
3. Kollias A., Kyriakoulis K.G., Dimakakos E. et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–7. https://doi.org/10.1111/ bjh.16727.
4. Gashimova N.R., Bitsadze V.O., Pankratyeva L.L. et al. Dysregulated platelet function in COVID-19 patients. [Disregulyaciya funkcii trombocitov u bol'nyh COVID-19]. Obstetrics, Gynecology and Reproduction. 2022;16(6):692–705. (In Russ.). https://doi.org/10.17749/2313-7347/ ob.gyn.rep.2022.372.
5. Bitsadze V.O., Slukhanchuk E.V., Khizroeva J.Kh. Extracellular neutrophil traps (NETs) in the pathogenesis of thrombosis and thromboinflammation. [Vnekletochnye lovushki nejtrofilov (NETs) v patogeneze tromboza i trombovospalitel'nyh zabolevanij]. Vestnik RAMN. 2021;76(1):75–85. (In Russ.). https://doi.org/10.15690/vramn1395.
6. Gibson P.G., Qin L., Puah S.H. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID19 ARDS. Med J Aust. 2020;213(2):54–56.e1. https://doi.org/10.5694/mja2.50674.
7. Ribes A., Vardon-Bounes F., Mémier V. et al. Thromboembolic events and Сovid-19. Adv Biol Regul. 2020;77:100735. https://doi.org/10.1016/j. jbior.2020.100735.
8. Cardona-Perez ́ J.A., Villegas-Mota I., Helguera-Repetto A.C. et al. Prevalence, clinical features, and outcomes of SARS-CoV-2 infection in pregnant women with or without mild/moderate symptoms: results from universal screening in a tertiary care center in Mexico City, Mexico. PLoS One. 2021;16(4):e0249584. https://doi.org/10.1371/journal.pone.0249584.
9. Martinelli I., Ferrazzi E., Ciavarella A. et al. Pulmonary embolism in a young pregnant woman with COVID-19. Thromb Res. 2020;191:36–7. https://doi.org/10.1016/j.thromres.2020.04.022.
10. Varga Z., Flammer A.J., Steiger P. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. https://doi.org/10.1016/S0140-6736(20)30937-5.
11. Makatsariya A.D., Slukhanchuk E.V., Bitsadze V.O. et al. Thrombotic microangiopathy, DIC-syndrome and COVID-19: link with pregnancy prothrombotic state. J Matern Neonatal Med. 2022;35(13):2536–44. https://doi.org/10.1080/14767058.2020.1786811.
12. Tiscia G., Favuzzi G., De Laurenzo A. et al. The prognostic value of adamts-13 and von willebrand factor in covid-19 patients: prospectiveevaluation by care setting. Diagnostics (Basel). 2021;11(9):1648. https://doi.org/10.3390/diagnostics11091648.
13. Bitsadze V.O., Khizroeva J.Kh., Gris J.-C. et al. Pathogenetic and prognostic significance of inflammation and altered ADAMTS-13/vWF axis in patients with severe COVID-19. [Patogeneticheskoe i prognosticheskoe znachenie vospaleniya i narushenij v osi ADAMTS-13/vWF u bol'nyh tyazheloj formoj COVID-19]. Obstetrics, Gynecology and Reproduction. 2022;16(3):228–43. (In Russ.). https://doi.org/10.17749/2313-7347/ob. gyn.rep.2022.327.
14. Fogarty H., Townsend L., Morrin H. et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19(10):2546–53. https://doi.org/10.1111/jth.15490.
15. Bonaventura A., VecchiéA., Dagna L. et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–29. https://doi.org/10.1038/s41577-021- 00536-9.
16. Grandone E., Vimercati A., Sorrentino F. et al. Obstetric outcomes in pregnant COVID-19 women: the imbalance of von Willebrand factor and ADAMTS13 axis. BMC Pregnancy Childbirth. 2022;22(1):142. https://doi. org/10.1186/s12884-022-04405-8.
About the Authors
N. R. GashimovaRussian Federation
Nilufar R. Gashimova – MD, Postgraduate Student, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
K. N. Grigoreva
Russian Federation
Kristina N. Grigoreva – MD, Assistant, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
V. O. Bitsadze
Russian Federation
Victoria O. Bitsadze – MD, Dr Sci Med, Professor of RAS, Professor, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 6506003478
Researcher ID: F-8409-2017
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
L. L. Pankratyeva
Russian Federation
Liudmila L. Pankratyeva – MD, Dr Sci Med, Associate Professor, Professor, Department of Pediatrics and Health Organization; Neonatologist, Hematologist, Head of the Clinical Research Center
1 Samora Machel Str., Moscow 117997
2/44 Salyama Adilya Str., Moscow 123423
J. Kh. Khizroeva
Russian Federation
Jamilya Kh. Khizroeva – MD, Dr Sci Med, Professor, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 57194547147
Researcher ID: F-8384-2017
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
M. V. Tretyakova
Russian Federation
Maria V. Tretyakova – MD, PhD, Obstetrician-Gynecologist, Assistant, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Ya. M. Shammut
Russian Federation
Yana M. Shammut – Student, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
E. Iu. Iupatov
Russian Federation
Evgenii Iu. Iupatov – MD, PhD, Associate Professor, Head of the Department of Obstetrics and Gynecology; Associate Professor, Department of Surgery and Postgraduate Education, Institute of Fundamental Medicine and Biology
Scopus Author ID: 57201192778
36 Butlerova Str., Kazan 420015
18 Kremlevskaya Str., Kazan 420008
V. I. Tsibizova
Russian Federation
Valentina I. Tsibizova – MD, PhD, Obstetrician-Gynecologist, Research Laboratory of Operative Gynecology, Institute of Perinatology and Pediatrics
2 Akkuratova Str., Saint Petersburg 197341
J.-K. Gris
Russian Federation
Jean-Christophe Gris – MD, Dr Sci Med, Professor, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health; Professor of Haematology, Head of the Laboratory of Haematology, Faculty of Biological and Pharmaceutical Sciences, Montpellier University and University Hospital of Nîmes; Foreign Member of RAS
Scopus Author ID: 7005114260
Researcher ID: AAA-2923-2019
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
163 Rue Auguste Broussonnet, Montpellier 34090, France
D. V. Blinov
Russian Federation
Dmitry V. Blinov – MD, PhD, MBA, Assistant, Department of Sports Medicine and Medical Rehabilitation, Sklifosovsky Institute of Clinical Medicine; Head of Medical and Scientific Affairs; Associate Professor, Department of Sports, Physical and Rehabilitation Medicine
Scopus Author ID: 6701744871
Researcher ID: E-8906-2017
RSCI: 9779-8290
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
4–10 Sadovaya-Triumfalnaya Str., Moscow 127006
5 bldg. 1–1a, 2-ya Brestskaya Str., Moscow 123056
A. D. Makatsariya
Russian Federation
Alexander D. Makatsariya – MD, Dr Sci Med, Academician of RAS, Professor, Head of the Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 57222220144
Researcher ID: M-5660-2016
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Review
For citations:
Gashimova N.R., Grigoreva K.N., Bitsadze V.O., Pankratyeva L.L., Khizroeva J.Kh., Tretyakova M.V., Shammut Ya.M., Iupatov E.I., Tsibizova V.I., Gris J., Blinov D.V., Makatsariya A.D. Clinical significance of assessing ADAMTS-13 and von Willebrand factor level in COVID-19 convalescent pregnant women. Obstetrics, Gynecology and Reproduction. 2023;17(1):8-17. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.386

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































