Перейти к:
Роль Wnt/β-катенинового сигнального пути в патогенезе эндометриоза
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2025.640
Аннотация
Эндометриоз – это хроническое эстрогензависимое заболевание, характеризующееся эктопическим ростом ткани, подобной эндометрию, за пределами матки. В последние годы все большее внимание уделяется роли Wnt/β-катенинового сигнального пути в патогенезе эндометриоза. Данный путь участвует в регуляции клеточной пролиферации, миграции, инвазии и фиброзных процессов. Гиперактивация Wnt/β-катенинового каскада способствует прогрессированию заболевания, хроническому воспалению и развитию спаечного процесса. Особое значение имеют микроРНК, регулирующие этот сигнальный путь. Изучение новых звеньев патогенеза эндометриоза открывает перспективы для неинвазивной диагностики и таргетной терапии. Дальнейшие исследования могут способствовать разработке новых методов лечения и улучшению качества жизни пациенток.
Ключевые слова
Для цитирования:
Виривская Е.В., Игнатко И.В., Снимщикова И.А., Бахтияров К.Р., Зуева А.С., Капырина Т.Д. Роль Wnt/β-катенинового сигнального пути в патогенезе эндометриоза. Акушерство, Гинекология и Репродукция. 2025;19(4):534-544. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2025.640
For citation:
Virivskaya E.V., Ignatko I.V., Snimshchikova I.A., Bakhtiyarov K.R., Zueva A.S., Kapyrina T.D. Wnt/β-catenin signaling pathway role in the endometriosis pathogenesis. Obstetrics, Gynecology and Reproduction. 2025;19(4):534-544. (In Russ.) https://doi.org/10.17749/2313-7347/ob.gyn.rep.2025.640
Введение / Introduction
Эндометриоз – хроническое распространенное эстрогензависимое заболевание, характеризующееся эктопическим ростом ткани, подобной эндометрию, за пределами матки [1]. Это доброкачественный процесс, способный проявлять свойства, родственные злокачественным опухолям: имплантация, инвазия и формирование отдаленных метастазов, за что был назван «доброкачественным раком» в гинекологии [2].
В последние годы заболеваемость эндометриозом постепенно увеличивается. По данным Всемирной организации здравоохранения (ВОЗ), эндометриоз затрагивает примерно 10 % женщин репродуктивного возраста по всему миру, что составляет около 190 млн человек [3]. В России насчитывается примерно 2 млн женщин, страдающих этим заболеванием [4].
Эндометриоз значительно снижает качество жизни женщин. Основными клиническими проявлениями эндометриоза являются хроническая тазовая боль, дисменорея, бесплодие, дисхезия, диспареуния, а также нарушения менструального цикла и аномальные маточные кровотечения. У пациенток, страдающих дисменореей и хроническими тазовыми болями, частота выявления эндометриоза варьирует от 35 до 50 % [5].
Эндометриоз – полиэтиологическое заболевание, патогенез которого остается предметом активных научных дискуссий. На основании анализа современных исследований можно выделить 6 ключевых концепций, каждая из которых вносит вклад в понимание механизмов развития патологии. Одной из ключевых гипотез патогенеза является теория ретроградной менструации J.A. Sampson [6]. Однако существует множество данных, сообщающих о многофакторных причинах возникновения эндометриоза, включая эктопию эндометриальной ткани, иммунные реакции, нарушение пролиферации клеток и апоптоза, аберрантную эндокринную сигнализацию и генетические факторы [7].
В последнее время особое внимание уделяется изучению Wnt-сигнальных путей в патогенезе эндометриоза. Wnt-сигнальные пути представляют собой эволюционно высококонсервативные механизмы межклеточной коммуникации, играющие ключевую роль в регуляции многочисленных физиологических процессов организма [8]. Они участвуют в основных биологических процессах, включая эмбриональное развитие, органогенез, тканевой гомеостаз тканей, функционирование стволовых клеток во взрослом организме, а также их дифференцировку [9]. Это сложная белковая сеть, состоящая из 19 гликопротеинов, которые взаимодействуют с рецепторами на поверхности клеток. Основными компонентами Wnt-сигнальных каскадов являются: лиганды Wnt; рецепторы белка FzD (англ. Frizzled), связывающие Wnt-лиганды и активирующие сигнальные каскады; корецепторы, например, белки, родственные рецептору липопротеинов низкой плотности 5-го и 6-го типа (англ. low-density lipoprotein receptor-related protein 5/6, LRP5/6); внутриклеточные медиаторы, например, Dsh (dishevelled, β-катенин); транскрипционные факторы, например, T-клеточный фактор/лимфоидный усилитель-связывающий фактор (англ. T-cell factor/lymphoid enhancer-binding factor, TCF/LEF) [10].
Wnt-сигналинг регулирует пролиферацию, дифференцировку и миграцию клеток через канонический (β-катенин-зависимый) и неканонические пути. Неканонические пути могут включать Wnt/PCP (англ. planar cell polarity; Wnt-сигнальный путь, регулирующий полярность клеток в плоскости ткани) и Wnt/Ca2+-зависимый сигналинг, которые влияют на клеточную морфологию и подвижность [11]. Канонический путь (β-катенин-зависимый) характеризуется стабилизацией β-катенина и его последующей транслокацией в ядро, где он регулирует экспрессию генов-мишеней [9]. Именно он играет ключевую роль в развитии и прогрессировании эндометриоза.
Wnt/β-катениновый путь / Wnt/β-catenin pathway
Исследования показывают, что гиперактивация Wnt/β-катенинового пути способствует формированию эндометриоидных очагов и поддержанию воспалительного процесса в пораженных тканях. В отсутствие лиганда Wnt цитоплазматический β-катенин поддерживается на низком уровне за счет действия комплекса деградации. Связавшись с комплексом деградации, который состоит из белка-супрессора опухолей аденоматозного полипоза coli (англ. аdenomatous рolyposis сoli, АРС), каркасного белка – аксиального ингибитора (англ. axial inhibitor protein, Axin) и двух киназ – казеинкиназы 1α (англ. сasein kinase 1α, CK1α) и киназы гликогенсинтазы-3β (англ. glycogen synthase kinase-3β, GSK-3β), β-катенин не проникает в клеточное ядро, ингибируя тем самым сигнальные каскады. Однако при связывании лиганда Wnt с рецептором FzD и корецептором LRP5/6 компоненты комплекса деградации перемещаются к клеточной мембране, делая ее неактивной. Этот процесс приводит к диссоциации β-катенина от комплекса деградации, и как следствие, к его накоплению и стабилизации в цитоплазме [12]. Затем β-катенин транслоцируется в ядро, где взаимодействует с семейством факторов TCF/LEF, инициируя процесс транскрипции целевых генов [13] (рис. 1).
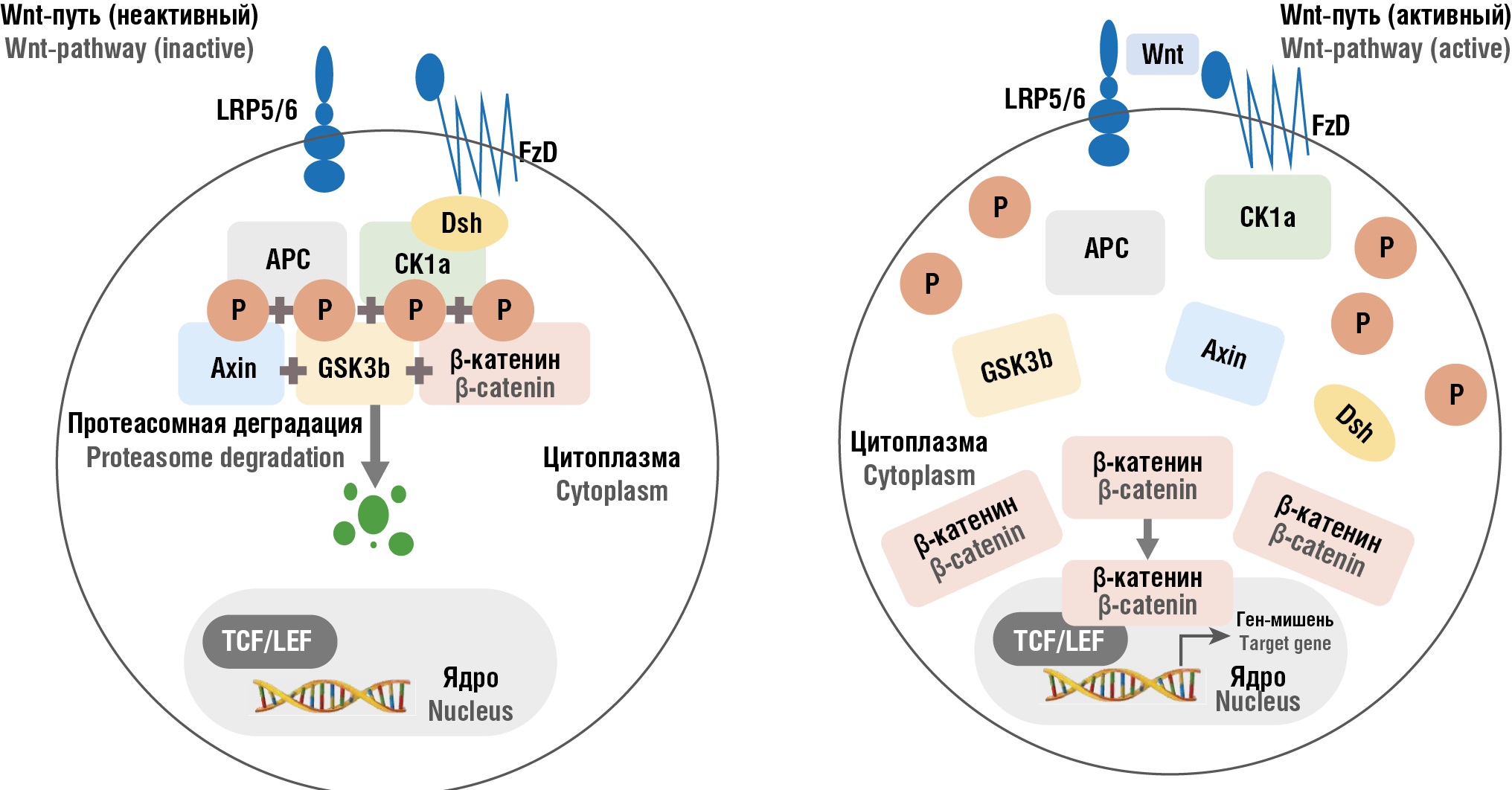
Рисунок 1. Механизм активации Wnt/β-катенинового сигнального пути [рисунок авторов].
Примечание: Wnt – сигнальные молекулы семейства Wnt, активирующие каскад при связывании с рецепторами; FzD (Frizzled) – рецепторы, воспринимающие сигнал Wnt на поверхности клетки; LRP5/6 – белки, родственные рецептору липопротеинов низкой плотности 5-го и 6-го типа, корецепторы, необходимые для запуска внутриклеточной передачи сигнала; Dsh (dishevelled) – внутриклеточный белок, активируемый при взаимодействии Wnt с рецепторами, тормозит деградацию β-катенина; CK1α – казеинкиназа 1α, фермент, участвующий в фосфорилировании β-катенина в составе деструктивного комплекса; GSK3β – гликогенсинтаза киназа-3β, протеинкиназа, способствующая фосфорилированию и деградации β-катенина; Axin – адаптерный белок, формирующий деструктивный комплекс для β-катенина; APC – белок-супресор опухолей аденоматозного полипоза, компонент комплекса, регулирующего деградацию β-катенина; β-катенин – ключевой медиатор пути Wnt, при активации пути накапливается и транслоцируется в ядро, где участвует в регуляции транскрипции; TCF/LEF – T-клеточный фактор/лимфоидный усилитель-связывающий фактор, транскрипционные факторы, активируемые β-катенином; P – фосфатная группа, указывающая на процесс фосфорилирования белков как ключевого регуляторного механизма, способствующего деградации β-катенина в неактивном состоянии Wnt/β-катенинового сигнального пути.
Wnt-путь (неактивный): при отсутствии лиганда Wnt/β-катенин подвергается фосфорилированию в составе комплекса деструкции (Axin, APC, GSK3β, CK1α), что приводит к его протеасомной деградации.
Wnt-путь (активный): связывание Wnt с рецепторным комплексом FzD и ко-рецептором LRP5/6 приводит к активации Dsh, дестабилизации деструктивного комплекса и накоплению β-катенина в цитоплазме. Последний транслоцируется в ядро, где взаимодействует с транскрипционными факторами TCF/LEF и запускает экспрессию генов-мишеней.
Figure 1. Mechanism of Wnt/β-catenin signaling pathway activation [drawn by authors].
Note: Wnt – signaling molecules of the Wnt family that activate the cascade upon binding to cognate receptors; FzD (Frizzled) – receptors sensing Wnt signal on the cell surface; LRP5/6 – low-density lipoprotein receptor-related proteins 5 and 6, coreceptors essential for initiating intracellular signal transduction; Dsh (dishevelled) – an intracellular protein activated upon Wnt-receptor interaction; inhibits β-catenin degradation; CK1α – casein kinase 1α, an enzyme involved in phosphorylating β-catenin as part of the destruction complex; GSK3β – glycogen synthase kinase-3β, a protein kinase that promotes β-catenin phosphorylation and degradation; Axin (axial inhibitor) – an adaptor protein forming the destruction complex for β-catenin; APC – adenomatous polyposis coli tumor suppressor protein, a component of the complex regulating β-catenin degradation; β-catenin – the key mediator of the Wnt-pathway; upon pathway activation, it undergoes accumulation and nuclear translocation to regulate transcription; TCF/LEF – T-cell factor/lymphoid enhancer-binding factor, transcription factors activated by β-catenin; P – phosphate group, indicating protein phosphorylation as a key regulatory mechanism promoting β-catenin degradation in the inactive Wnt/β-catenin signaling pathway.
Wnt-pathway (inactive): in the absence of Wnt ligand, β-catenin undergoes phosphorylation within the destruction complex (Axin, APC, GSK3β, CK1α), leading to its proteasomal degradation.
Wnt-pathway (active): binding of Wnt to the FzD receptor complex and the LRP5/6 co-receptor activates Dsh, destabilizes the destruction complex, and leads to β-catenin accumulation in the cytoplasm. β-catenin then translocates into the nucleus, where it interacts with TCF/LEF transcription factors and initiates target gene expression.
Была выявлена взаимосвязь между патологической активацией Wnt/β-катенинового пути и прогрессированием различных онкологических заболеваний, таких как рак мочевого пузыря, плоскоклеточный рак ротовой полости, колоректальный рак и др. [14]. Дальнейшие исследования показали, что злокачественные биологические характеристики эндометриоза также связаны с аберрантной активацией сигнального пути Wnt/β-катенина. Так, например, S. Matsuzaki с соавт. еще в 2013 г. выявили значительное повышение уровня β-катенина в эктопическом эндометрии, что способствует инвазии клеток и их устойчивости к апоптозу [15] (рис. 1).
Основные механизмы нарушения регуляции Wnt/β-катенинового пути / Basic mechanisms of Wnt/β-catenin pathway dysregulation
Путь Wnt включает в себя многочисленные внеклеточные и внутриклеточные регуляторные белки, принадлежащие к двум функциональным классам. Первые связываются с Wnt и активируют белки семейства SFRP (англ. secreted Frizzled-related protein; Frizzled-связанный секретируемый белок); вторые взаимодействуют с белками семейства Dickkopf (DKK) – семейство секретируемых белков, ингибирующих Wnt-сигнальный путь путем связывания с корецепторами LRP5/6, подавляя тем самым сигнализацию Wnt [16].
Одним из механизмов нарушения регуляции Wnt/β-катенинового пути является подавление ингибитора DKK-1 и инактивация GSK-3β. В исследовании, проведенном в 2018 г. Р. Pazhohan с соавт., сравнивали эутопический эндометрий и перитонеальные эндометриоидные очаги у пациенток с тяжелым эндометриозом (стадии III–IV) со здоровой тканью эндометрия в середине секреторной фазы менструального цикла. Уровни матричной РНК (мРНК) и белка DKK-1 (ингибитора пути Wnt) были значительно снижены как в эутопическом эндометрии, так и в эндометриоидных очагах пациенток по сравнению со здоровым контролем. Различий между эутопическими и эктопическими тканями в группе эндометриоза не наблюдалось. Уровни активного (нефосфорилированного) β-катенина были повышены в тканях эндометриоза, в то время как экспрессия общего β-катенина оставалась неизменной. Уровни фосфорилированного (неактивного) GSK-3β были выше в тканях эндометриоза, что указывает на сниженную способность к деградации β-катенина [17]. Эти молекулярные изменения способствуют усиленной клеточной пролиферации, формированию резистентности к прогестерону.
В 2018 г. Т. Heinosalo с соавт. провели анализ экспрессии генов в образцах эутопического эндометрия, а также в образцах эндометриоидных очагов у 103 пациенток с эндометриозом, в качестве группы контроля была взята биопсия эндометрия из полости матки у 47 здоровых женщин. Исследование показало, что у пациентов с наружным генитальным эндометриозом (неяичниковой формы) значительно увеличивается экспрессия белка SFRP2, что указывает на его роль в патогенезе заболевания. Отмечалась прямая корреляция экспрессии SFRP2 с симптомами боли у пациентов [18]. Считается, что деметилирование ДНК, регулирующего промоутер SFRP, приводит к его экспрессии SFRP2 и тем самым активирует путь Wnt/β-катенина, способствуя инвазии и миграции эктопического эндометрия [19]. Исходя из полученных данных, можно сделать вывод, что усиление сигнала Wnt/β-катенина, вызванное экспрессией SFRP2 вследствие деметилирования промотора SFRP2, играет важную роль в патогенезе эндометриоза, а также не исключает возможности использования SFRP2 в качестве терапевтической мишени для лечения (рис. 2).
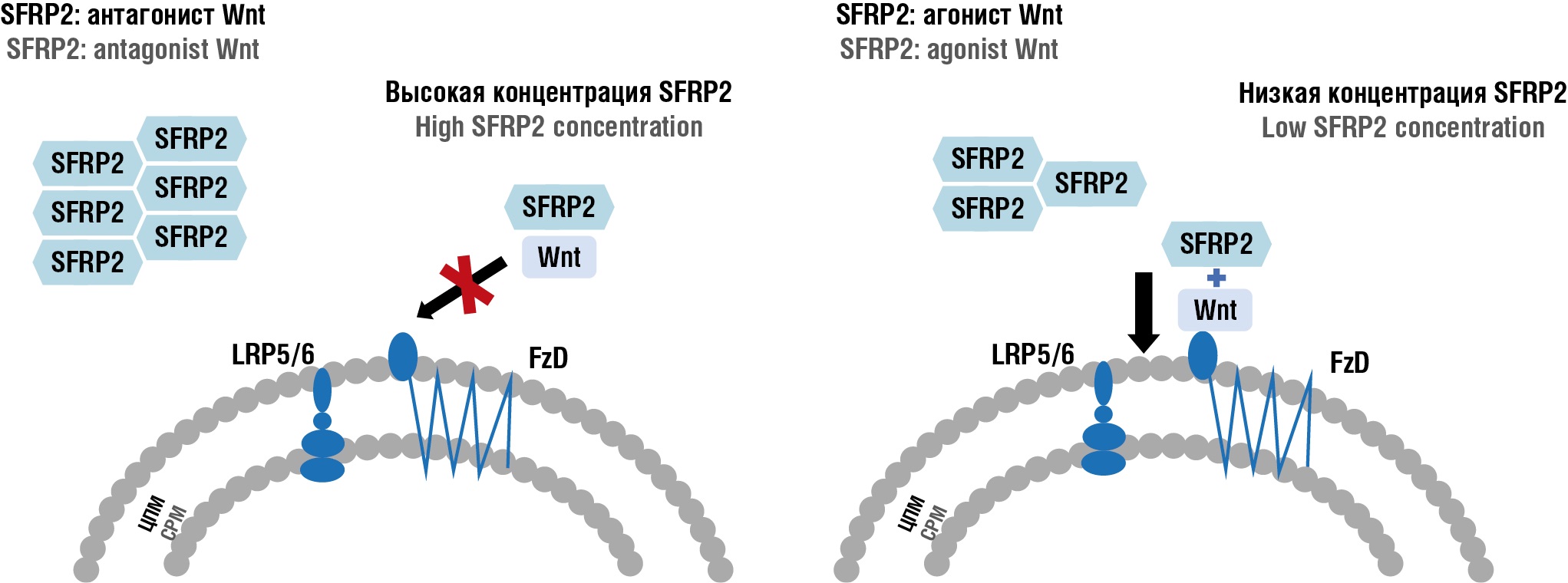
Рисунок 2. Роль SFRP2 в регуляции Wnt-сигнального пути в зависимости от его концентрации [рисунок авторов].
Примечание: Wnt – сигнальные молекулы семейства Wnt, активирующие каскад при связывании с рецепторами; FzD (Frizzled) – рецепторы, воспринимающие сигнал Wnt на поверхности клетки; LRP5/6 – белки, родственные рецептору липопротеинов низкой плотности 5-го и 6-го типа, ко-рецепторы, необходимые для запуска внутриклеточной передачи сигнала; ЦМП – цитоплазматическая мембрана; SFRP2 – Frizzled-связанный секретируемый белок, регулирующий активность Wnt-сигнального пути; может действовать как антагонист или агонист в зависимости от концентрации; SFRP2 – как антагонист Wnt, при высокой концентрации молекулы SFRP2 связывают Wnt-лиганды и препятствуют их взаимодействию с рецепторным комплексом FzD/LRP5/6, блокируя активацию сигнального каскада; SFRP2 – как агонист Wnt, при низкой концентрации SFRP2 происходит формирование комплекса SFRP2–Wnt, способствует стабилизации Wnt-лиганда и облегчает его связывание с рецепторным комплексом, что приводит к активации Wnt-сигнального пути.
Figure 2. Dose-dependent SFRP2 role in regulating the Wnt-signaling pathway [drawn by authors].
Note: Wnt – signaling molecules of the Wnt family that activate the cascade upon binding to receptors; FzD (Frizzled) – receptors sensing Wnt signals on the cell surface; LRP5/6 – density lipoprotein receptor-related proteins 5 and 6, co-receptors essential for initiating intracellular signal transduction; CMP – cytoplasmic membrane; SFRP2 (Secreted Frizzled-Related Protein 2) – a Frizzled-associated secreted protein that regulates Wnt-signaling pathway activity; can function as either an antagonist or agonist depending on concentration; SFRP2 as Wnt antagonist: at high concentrations, SFRP2 molecules bind Wnt-ligands and sense their interaction with the FzD/LRP5/6 receptor complex, thereby blocking signal cascade activation; SFRP2 as Wnt agonist: at low concentrations, SFRP2 forms SFRP2-Wnt complexes that stabilize Wnt-ligands and facilitate their binding to the receptor complex, leading to Wnt-pathway activation.
Эстрогены играют ключевую роль в патогенезе эндометриоза. Они могут активировать сигнальные пути Wnt/β-катенина, способствуя пролиферации клеток и нарушая нормальные процессы апоптоза. Исследования показывают, что под влиянием 17β-эстрадиола (E2) активируется экспрессия генов матриксной металлопротеиназы (англ. matrix metalloproteinase, ММР) MMP-9 и фактора роста эндотелия сосудов (англ. vascular endothelial growth factor, VEGF) через сигнальный путь Wnt/β-катенина. Ремоделирование внеклеточного матрикса необходимо для имплантации эктопического эндометрия. Данный процесс приводит к его инвазии и миграции клеток, а также активирует процессы ангиогенеза [20][21]. Кроме того, известно, что под действием эстрогенов происходит значительное снижение экспрессии белка Dickkopf-1 (DKK-1) и, как следствие, активация пути Wnt [17]. Прогестерон, напротив, активирует DKK1, ингибируя Wnt-сигналинг в эндометрии [22].
Также в одном из исследований сообщалось, что снижение экспрессии Е-кадгерина (англ. сadherin 1, E-cadherin, CDH-1) – гликопротеина, необходимого для межклеточной адгезии, приводит к цитоплазматическому накоплению β-катенина, активируя тем самым Wnt-сигнальный путь [23]. Е-кадгерин играет важную роль в процессах метастазирования, соответственно снижение его активности может усилить миграцию и инвазию клеток, провоцируя развитие эндометриоза.
При изучении патогенеза эндометриоза исследователи уделяют особое внимание CTHRC1 (англ. collagen triple helix repeat containing 1) – секретируемому белку внеклеточного матрикса, играющему ключевую роль в регуляции клеточной миграции, инвазии и ремоделировании тканей. Его аномальная экспрессия ассоциирована с прогрессированием различных злокачественных опухолей. В последние годы была выявлена связь CTHRC1 с активацией Wnt-сигнальных путей [24][25].
По полученным данным, CTHRC1 повышает стабильность и транскрипционную активность β-катенина, способствуя его ядерной транслокации [26]. Также CTHRC1 взаимодействует с компонентами неканонического Wnt/PCP-пути, стабилизируя комплекс Wnt5a/Frizzled/Ror2 и активируя RhoA (англ. Ras homolog family member A) – малую гуанозиндифосфатазу подсемейства Ras-белков, играющую ключевую роль в организации актинового цитоскелета и клеточной миграции, и JNK (англ. c-Jun N-terminal kinase; семейство стресс-активируемых протеинкиназ). Хотя в патогенезе эндометриоза доминирует β-катенин-зависимый механизм, Wnt/PCP-сигналинг может участвовать в регуляции клеточной миграции [27].
Установлено, что уровень CTHRC1 значительно повышен в эктопических очагах эндометрия по сравнению с эутопическим эндометрием. Сывороточная концентрация CTHRC1 также выше у пациенток с эндометриозом, что позволяет рассматривать его как диагностический маркер [28]. Кроме того, CTHRC1 ингибирует фосфорилирование GSK-3β, предотвращая деградацию β-катенина, повышает экспрессию MMP-2, MMP-9 и снижает уровень E-кадгерина [24]. Было изучено взаимодействие CTHRC1 с интегринами (например, β3), посредством которого активируются сигнальные каскады FAK/Akt (англ. focal adhesion kinase/protein kinase B; киназа фокальных адгезий/протеинкиназа B) – ключевые сигнальные молекулы, участвующие в адаптации, пролиферации клеток, опосредующие их миграцию и запуск метастатических процессов [29].
В качестве маркера эндометриоза можно выделить MRP4 (англ. multi-drug resistance-associated protein 4) – транспортный белок, играющий важную роль в поддержании Wnt/β-катенинового сигнального пути в эндометрии. Исследования демонстрируют, что MRP4 взаимодействует с β-катенином, предотвращая его деградацию через протеосомный путь. В исследовании J.-J. Chen с соавт. продемонстрирована положительная корреляция между повышенной экспрессией MRP4 и уровнем β-катенина в эндометриоидных очагах (r = 0,25; p < 0,01) [30]. Также выявлена значимая связь между экспрессией MRP4 и генами Wnt-пути, например, CCND1 (англ. Cyclin D1; циклин D1), IL-6 (англ. interleukin; интерлейкин) при эндометриозе и раке эндометрия [31].
Формирование спаечного процесса при эндометриозе / Formation of adhesions in endometriosis
Одной из проблем, затрагивающих пациентов с наружным генитальным эндометриозом (НГЭ), является развитие спаечного процесса в малом тазу. Считается, что данное осложнение возникает в 60 % случаев НГЭ, вызывая синдром хронической тазовой боли [32].
Современные исследования показывают, что эндометриоидный процесс аналогичен многократному циклическому процессу повреждения и восстановлению тканей, что неизбежно приводит к ее фиброзированию.
Так, S.W. Guo в своей статье утверждает, что фиброгенез является ключевым аспектом, лежащим в основе эндометриоза. Автор описывает процессы, происходящие в эндометриоидных очагах, включая переход эпителия в мезенхиму и трансдифференцировку фибробластов в миофибробласты, что приводит к образованию фиброзной ткани. Этим можно объяснить устойчивость к традиционным гормональным методам лечения [33].
Известно, что избыточная активация сигнальных путей Wnt/β-катенина может приводить к фиброзу в эндометриоидных тканях. Механически сигнальный путь Wnt регулирует экспрессию генов фиброзных маркеров, включая фактор роста соединительной ткани (англ. connective tissue growth factor, CTGF), коллаген I типа (англ. collagen type I, Col-I), α-гладкомышечный актин (англ. α-smooth muscle actin, αSMA) и фибронектин (англ. fibronectin, FN), каждый из которых участвует в фиброгенезе эндометриоидных тканей [15].
FOXP1 (англ. forkhead box protein 1) – фактор транскрипции, участвующий в развитии, поддержании гомеостаза, а также в регенерации взрослых тканей при различных заболеваниях [34]. Исследования демонстрируют что экспрессия гена FOXP1 повышается в эндометриоидных стромальных клетках. Известно, что FOXP1 участвует в процессе формирования фиброза тканей через сигнальный путь Wnt у пациентов с эндометриозом. FOXP1 действует как активатор сигнализации Wnt, способствуя ацетилированию β-катенина [35].
За формирование фиброза также отвечает протеинкиназа WEE1 (англ. WEE1 G2 checkpoint kinase; WEE1-киназа G2-контрольной точки) – ключевой регулятор клеточного цикла, фосфорилирующий циклин-зависимую киназу-1 (англ. сyclin-dependent kinase 1, CDK1) для предотвращения преждевременного вступления клеток в митоз. По некоторым данным, WEE1 повышается в эндометриальных стромальных клетках во время воспаления, вызванного IL-1β, что приводит к активации пути Wnt/β-катенина, играющего важную роль в патогенезе эндометриоза. Увеличение уровней маркеров α-SMA и Col-I при избыточной экспрессии WEE1 подтверждает его роль в развитии фиброза тканей [36].
В исследовании Y. Liu с соавт. подчеркивается ключевое значение эндометриальных мезенхимальных стволовых клеток (ЭМСК) в патогенезе эндометриоза [37]. Известно, что паракринная секреция трансформирующего фактора роста-β1 (англ. transforming growth factor-beta, TGF-β1) и Wnt1 ЭМСК активирует Wnt/β-катениновый сигнальный путь в стромальных клетках, индуцируя избыточное отложение внеклеточного матрикса и прогрессирование фиброза [38].
Взаимодействие между микроРНК и Wnt/β-катениновым сигнальным путем / Interaction between miRNAs and Wnt/β-catenin signaling pathway
Современные научные данные подтверждают значимое влияние микроРНК (англ. microRNA, miRNA) на молекулярные механизмы развития эндометриоза, уделяя особое внимание их взаимодействию с сигнальным каскадом Wnt/β-катенина [39]. МикроРНК активируют или ингибируют каскад сигнализации Wnt/β-катенинового пути посредством воздействия на ключевые компоненты сигнальной системы – от мембранных рецепторов до ядерных транскрипционных факторов. Этот эпигенетический регуляторный механизм оказывает существенное влияние на патогенез инициирования и прогрессирования различных заболеваний и неопластических процессов.
Анализ последних исследований продемонстрировал комплексную сеть взаимодействий молекул, функционирующих как транскрипционные регуляторы или прямые медиаторы экспрессии генов. Они координируют активность Wnt/β-катенин-опосредованных сигналов, часто с привлечением микроРНК [40].
МикроРНК модулируют активность Wnt/β-катенинового пути посредством нескольких механизмов.
Известно, что miR-33b подавляет транскрипционный фактор ZEB1 (англ. zinc finger E-box binding homeobox 1), угнетающий экспрессию E-кадгерина и участвующий в эпителиально-мезенхимальном переходе, снижая экспрессию Wnt и β-катенина и ингибируя рост и инвазию эндометриоидных клеток [41]. MiR-33b является ключевым регулятором как окисления жирных кислот, так и метаболизма холестерина [42]. Повышение уровня miR-33b подавляет пролиферацию опухолевых клеток, вызывает апоптоз, уменьшает инвазию и миграцию клеток, а также регулирует процесс эпителиально-мезенхимального перехода за счет повышения уровня E-кальмодулина при одновременном снижении уровня β-катенина [43]. Высокая экспрессия фактора транскрипции ZEB1 была продемонстрирована при различных злокачественных новообразованиях, включая рак молочной железы, простаты, легких и эндометрия [44]. Кроме того исследования показали, что ZEB1 участвует в эпигенетической регуляции эндометриоза, а также способствует формированию злокачественного фенотипа заболевания [45][46].
Было доказано, что внеклеточные везикулы (экзосомы) способны транспортировать микроРНК. Y. Zhang с соавт. считают, что экзосомы, секретируемые эктопическими эндометриальными стромальными клетками, могут ограничивать фиброз тканей посредством переноса miR-214 [47].
Особого внимания в патогенезе эндометриоза заслуживает miR-30c, доставляемая экзосомами эндометриальных эпителиальных клеток, она напрямую нацелена на белок B-клеточной лимфомы 9 (англ. B-cell lymphoma 9 protein, BCL9) – онкоген, транскрипционный коактиватор Wnt/β-катенинового сигнального пути. Инактивация BCL9 замедляет процесс метастазирования и ангиогенез посредством ингибирования экспрессии клеточного миелоцитоматозного онкогена (англ. cellular myelocytomatosis oncogene, c-Myc), циклина D1, СD44 (маркер клеточной адгезии и миграции), VEGF [48]. В исследовании M. Zhang с соавт. отмечено, что экспрессия генов miR-30 была заметно снижена в эктопических и эутопических эндометриоидных очагах [49]. Экзосомы, доставляющие miR-30c, могут быть использованы как потенциальная терапевтическая стратегия для лечения эндометриоза и других заболеваний, связанных с дисрегуляцией пути Wnt/β-катенина. Кроме того, miR-30c и BCL9 могут служить биомаркерами для прогнозирования и мониторинга заболеваний (рис. 3).
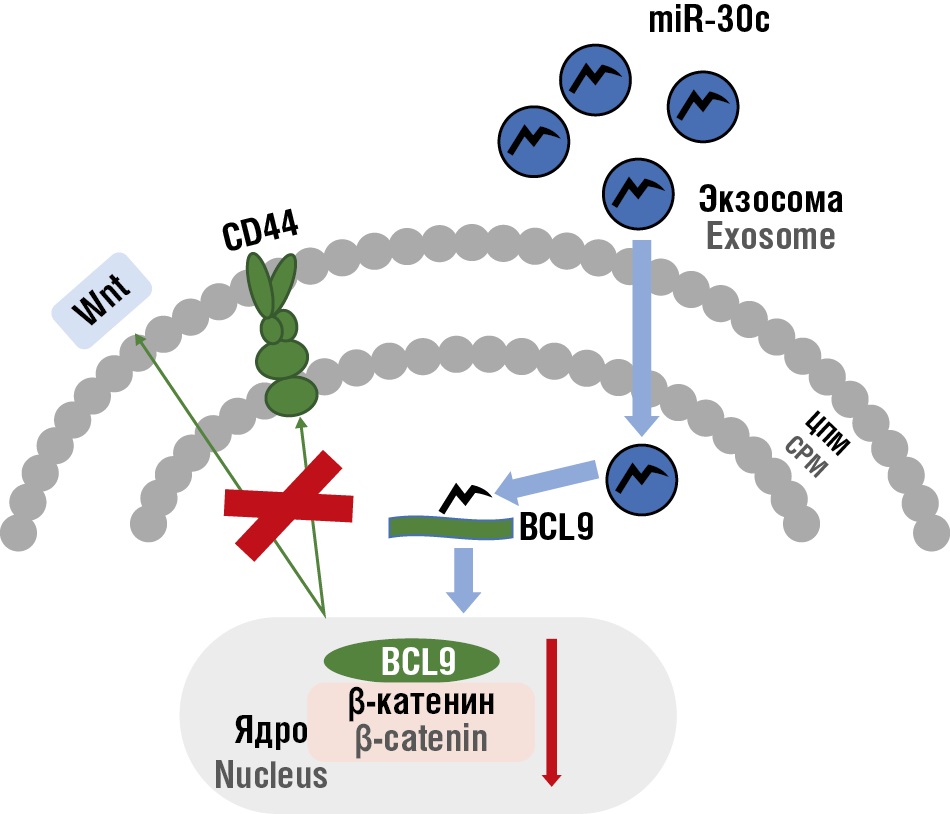
Рисунок 3. Механизм молекулярной регуляции сигнального пути Wnt/β-катенина посредством экзосомальных микроРНК-30c (miR-30c) в клетках-мишенях [рисунок авторов].
Примечание: Wnt – Wnt/β-катениновый сигнальный путь; BCL9 – белок B-клеточной лимфомы 9, коактиватор транскрипции в Wnt/β-катениновом сигнальном пути, участвующий в прогрессии опухолей и регуляции иммунного ответа; СD44 – маркер клеточной адгезии и миграции, выступает как ко-рецептор и модулятор Wnt-пути; ЦМП – цитоплазматическая мембрана.
Figure 3. Molecular mechanism of the Wnt/β-catenin signaling pathway regulation by exosomal microRNA-30c (miR-30c) in target cells [drawn by authors].
Note: Wnt – Wnt/β-catenin signaling pathway; BCL9 – B-cell lymphoma 9 protein, a transcriptional coactivator in the Wnt/β-catenin signaling pathway involved in tumor progression and immune response regulation; CD44 – cell adhesion and migration marker, functions as a co-receptor and modulator of the Wnt pathway; СMP – cytoplasmic membrane.
Активация рецептора CD44 под действием лиганда Wnt инициирует передачу сигнала, ведущую к экспрессии коактиватора BCL9, что способствует транслокации β-катенина в ядро и активации транскрипции соответствующих генов.
Экзосомы, содержащие miR-30c, попадают в клетку и подавляют экспрессию BCL9 на посттранскрипционном уровне. Это приводит к снижению уровня BCL9, что препятствует формированию комплекса BCL9/β-катенин в ядре и ингибирует активацию β-катенин-зависимых генов.
Еще одной микроРНК, дисрегуляция которой связана с патогенезом эндометриоза, является miR-488. Исследования показали, что miR-488 участвует в нескольких ключевых процессах, способствующих развитию и прогрессированию эндометриоза. Во-первых, аберрантная экспрессия miR-488 может способствовать эктопической имплантации эндометриальных клеток за счет усиления ангиогенеза, также способствуя ремоделированию внеклеточного матрикса [50]. В эксперименте на мышиной модели наблюдалось снижение экспрессии miR-488 в искусственном эктопическом эндометрии по сравнению с нормальной эндометриальной тканью. Повышение экспрессии miR-488 ингибировало путь Wnt/β-катенина посредством взаимодействия с FZD7 [51]. FZD7 – трансмембранный белок семейства FZD, а также наиболее распространенный рецептор Wnt, который может активировать как классический путь Wnt/β-катенина, так и неклассические сигнальные пути Wnt. FZD7 играет ключевую роль в регуляции пролиферации эндотелиальных клеток [52]. Из этого следует, что снижение экспрессии miR-488 способствует повышению регуляции каскада Wnt/β-катенина, тем самым облегчая инвазию эктопической эндометриальной ткани.
Изучение роли miR-488 в патогенезе эндометриоза открывает новые перспективы для диагностики и лечения данного заболевания; miR-488 – потенциальный биомаркер эндометриоза. Определение ее уровня в биологических жидкостях может использоваться для неинвазивной диагностики заболевания. Таргетная терапия, направленная на нормализацию экспрессии miR-488, может помочь подавить пролиферацию эктопического эндометрия и усилить апоптоз в очагах эндометриоза. Комбинация анализа экспрессии miR-488 с другими микроРНК позволит повысить точность диагностики и прогнозирования течения заболевания.
В патогенезе эндометриоза нельзя не выделить LINC01541 – это длинная некодирующая РНК (англ. long non-coding RNA, lncRNA), взаимодействующая с микроРНК. Путем снижения биодоступности miR-506-5p, LINC0154 модулирует сигнальный путь Wnt/β-катенина за счет посттранскрипционной регуляции экспрессии гена WIF1, а также ингибирует экспрессию VEGF-A [53][54]. Было установлено, что в клетках эндометриоидной аденокарциномы активность LINC01541 значительно подавляется под действием 17β-эстрадиола [54]. Таким образом, LINC01541 является важным регулятором клеточных процессов, участвующий в патогенезе эндометриоза, посредством взаимодействия с микроРНК и модуляции сигнальных путей.
Среди маркеров эндометриоза также выделяют Talin1 – важный цитоскелетный белок, играющий центральную роль в клеточной адгезии, механотрансдукции и активации интегринов. Избыточная экспрессия Talin-1 активирует путь Wnt/β-катенина. В исследовании Y.-Y. Wang с соавт. было выявлено, что экспрессия Talin-1 регулируется miR-145-5p. Talin-1 был заметно сверхэкспрессирован в эндометриальной ткани, тогда как miR-145-5p была снижена [55]. Полученные результаты иллюстрируют новые механизмы развития эндометриоза.
Заключение / Conclusion
Эндометриоз представляет собой сложное, полиэтиологическое заболевание, патогенез которого включает взаимодействие множества молекулярных механизмов. Особое внимание уделяется роли Wnt/β-катенинового сигнального пути, который участвует в регуляции клеточной пролиферации, миграции, инвазии и фиброгенеза. Исследования демонстрируют, что гиперактивация этого пути способствует прогрессированию эндометриоза, формированию хронического воспаления и развитию спаечного процесса.
Важной составляющей патогенеза являются микроРНК, которые модулируют активность Wnt/β-катенинового каскада. Эти молекулы открывают перспективы для разработки новых диагностических маркеров и таргетных терапевтических подходов. Например, miR-30c, miR-488 и другие микроРНК могут быть использованы для подавления патологической активности сигнального пути и предотвращения прогрессирования заболевания.
Несмотря на значительный прогресс в изучении патогенеза эндометриоза, многие аспекты остаются недостаточно изученными. Будущие исследования должны быть направлены на углубленное понимание взаимодействия между сигнальными путями, эпигенетическими механизмами и клеточными процессами. Это позволит разработать более эффективные методы диагностики и лечения, улучшая качество жизни пациенток с данным заболеванием.
Список литературы
1. Becker C.M., Bokor A., Heikinheimo O. et al. ESHRE guideline: endometriosis. Hum Reprod Open. 2022;2022(2):hoac009. https://doi.org/10.1093/hropen/hoac009.
2. Wilbur M.A., Shih I.M., Segars J.H., Fader A.N. Cancer implications for patients with endometriosis. Semin Reprod Med. 2017;35(1):110–6. https://doi.org/10.1055/s-0036-1597120.
3. Endometriosis. World Health Organization, 2023. Режим доступа: https://www.who.int/news-room/fact-sheets/detail/endometriosis. [Дата обращения: 25.02.2025].
4. Улумбекова Г.Э., Худова И.Ю. Оценка демографического, социального и экономического эффекта применения гормональной терапии при эндометриозе и аномальных маточных кровотечениях. ОРГЗДРАВ: новости, мнения, обучение. Вестник ВШОУЗ. 2022;8(1):82–113. https://doi.org/10.33029/2411-8621-2022-8-1-82-113.
5. Smolarz B., Szyłło K., Romanowicz H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int J Mol Sci. 2021;22(19):10554. https://doi.org/10.3390/ijms221910554.
6. Sampson J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol.1927;14:422–69. https://doi.org/10.1016/S0002-9378(15)30003-X.
7. Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–52. https://doi.org/10.1016/S0140-6736(21)00389-5.
8. Hayat R., Manzoor M., Hussain A. Wnt signaling pathway: a comprehensive review. Cell Biol Int. 2022;46(6):863–77. https://doi.org/10.1002/cbin.11797.
9. Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145(11):dev146589. https://doi.org/10.1242/dev.146589.
10. Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. https://doi.org/10.4161/org.4.2.5851.
11. Pataki C.A., Couchman J.R., Brábek J. Wnt signaling cascades and the roles of syndecan proteoglycans. J Histochem Cytochem. 2015;63(7):465–80. https://doi.org/10.1369/0022155415586961.
12. Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. https://doi.org/10.1186/s13045-020-00990-3.
13. Ranes M., Zaleska M., Sakalas S. et al. Reconstitution of the destruction complex defines roles of AXIN polymers and APC in β-catenin capture, phosphorylation, and ubiquitylation. Mol Cell. 2021;81(16):3246–3261. e11. https://doi.org/10.1016/j.molcel.2021.07.013.
14. Yu F., Yu C., Li F. et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):1–24. https://doi.org/10.1038/s41392-021-00701-5.
15. Matsuzaki S., Darcha C. Involvement of the Wnt/β-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS One. 2013;8(10):e76808. https://doi.org/10.1371/journal.pone.0076808.
16. Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–34. https://doi.org/10.1242/jcs.00623.
17. Pazhohan A., Amidi F., Akbari-Asbagh F. et al. The Wnt/β-catenin signaling in endometriosis, the expression of total and active forms of β-catenin, total and inactive forms of glycogen synthase kinase-3β, WNT7a and DICKKOPF-1. Eur J Obstet Gynecol Reprod Biol. 2018;220:1–5. https://doi.org/10.1016/j.ejogrb.2017.10.025.
18. Heinosalo T., Gabriel M., Kallio L. et al. Secreted frizzled-related protein 2 (SFRP2) expression promotes lesion proliferation via canonical WNT signaling and indicates lesion borders in extraovarian endometriosis. Hum Reprod. 2018;33(5):817–31. https://doi.org/10.1093/humrep/dey026.
19. Yang M., Li L., Huang X. et al. The DNA demethylation-regulated SFRP2 dictates the progression of endometriosis via activation of the Wnt/β-catenin signaling pathway. BMC Mol Cell Biol. 2023;24(1):12. https://doi.org/10.1186/s12860-023-00470-9.
20. Xu H., Yang J.J., Wang C.H. et al. Effect of Wnt/β-catenin signal pathway on of matrix metalloproteinase-7 and vascular endothelial growth factor gene expressions in endometriosis. Clin Exp Obstet Gynecol. 2016;43(4):573–7.
21. Zhang L., Xiong W., Xiong Y. et al. Intracellular Wnt/beta-catenin signaling underlying 17beta-estradiol-induced matrix metalloproteinase 9 expression in human endometriosis. Biol Reprod. 2016;94(3):70. https://doi.org/10.1095/biolreprod.115.135574.
22. Wang Y., Hanifi-Moghaddam P., Hanekamp E.E. et al. Progesterone inhibition of Wnt/β-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res. 2009;15(18):5784–93. https://doi.org/10.1158/1078-0432.CCR-09-0814.
23. Zhu X., Li Y., Zhou R. et al. Knockdown of E-cadherin expression of endometrial epithelial cells may activate Wnt/β-catenin pathway in vitro. Arch Gynecol Obstet. 2018;297(1):117–23. https://doi.org/10.1007/s00404-017-4560-0.
24. Yamamoto S., Nishimura O., Misaki K. et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15(1):23–36. https://doi.org/10.1016/j.devcel.2008.05.007.
25. Lv Y., Zhang L., Ma J. et al. CTHRC1 overexpression promotes ectopic endometrial stromal cell proliferation, migration and invasion via activation of the Wnt/β-catenin pathway. Reprod Biomed Online. 2020;40(1):26–32. https://doi.org/10.1016/j.rbmo.2019.10.001.
26. Liu Y.-J., Du J., Li J. et al. CTHRC1, a novel gene with multiple functions in physiology, disease and solid tumors (Review). Oncol Lett. 2023;25(6):266. https://doi.org/10.3892/ol.2023.13852.
27. Zhang R., Lu H., Lyu Y.Y. et al. E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis and activates Wnt/PCP pathway. Sci Rep. 2017;7:44744. https://doi.org/10.1038/srep44744.
28. Ruan F., Ma J., Xu K. Silencing of CTHRC1 inhibits proliferation and metastasis of endometriotic stromal cells. Int J Clin Exp Pathol. 2016;9(10):10028–35.
29. Hu X., Bian Y., Wen X. et al. Collagen triple helix repeat containing 1 promotes endometrial cancer cell migration by activating the focal adhesion kinase signaling pathway. Exp Ther Med. 2020;20(2):1405–14. https://doi.org/10.3892/etm.2020.8833.
30. Chen J.-J., Xiao Z.-J., Meng X. et al. MRP4 sustains Wnt/β-catenin signaling for pregnancy, endometriosis and endometrial cancer. Theranostics. 2019;9(1):5049–64. https://doi.org/10.7150/thno.32097.
31. De P., Aske J.C., Dale A. et al. Addressing activation of WNT beta-catenin pathway in diverse landscape of endometrial carcinogenesis. Am J Transl Res. 2021;13(11):12168–80.
32. Ярмолинская М.И., Адамян Л.В. Эндометриоз-ассоциированный болевой синдром и спаечный процесс – новые аспекты патогенеза и возможности терапии. Проблемы репродукции. 2023;29(2):93–100. https://doi.org/10.17116/repro20232902193.
33. Guo S.W. Fibrogenesis resulting from cyclic bleeding: the Holy Grail of the natural history of ectopic endometrium. Hum Reprod. 2018;33(3):353–6. https://doi.org/10.1093/humrep/dey015.
34. Katoh M., Igarashi M., Fukuda H. et al. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328(2):198–206. https://doi.org/10.1016/j.canlet.2012.09.017.
35. Shao X., Wei X. FOXP1 enhances fibrosis via activating Wnt/β-catenin signaling pathway in endometriosis. Am J Transl Res. 2018;10(11):3610–8.
36. Shi L., Xue X., Tian H. et al. WEE1 promotes endometriosis via the Wnt/β-catenin signaling pathway. Reprod Biol Endocrinol. 2021;19(1):161. https://doi.org/10.1186/s12958-021-00844-8.
37. Liu Y., Liang S., Yang F. et al. Biological characteristics of endometriotic mesenchymal stem cells isolated from ectopic lesions of patients with endometriosis. Stem Cell Res Ther. 2020;11(1): 346. https://doi.org/10.1186/s13287-020-01856-8.
38. Li J., Dai Y., Zhu H. et al. Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/β-catenin pathway by paracrine production of TGF-β1 and Wnt1. Hum Reprod. 2016;31(6):1224–35. https://doi.org/10.1093/humrep/dew058.
39. Zhang Y., Sun X., Li Z. et al. Interactions between miRNAs and the Wnt/β-catenin signaling pathway in endometriosis. Biomed Pharmacother. 2024;171:116182. https://doi.org/10.1016/j.biopha.2024.116182.
40. Cariello M., Squilla A., Piacente M. et al. Drug resistance: the role of exosomal miRNA in the microenvironment of hematopoietic tumors. Molecules. 2022;28(1):116. https://doi.org/10.3390/molecules28010116.
41. Zhang H., Li G., Sheng X., Zhang S. Upregulation of miR-33b promotes endometriosis via inhibition of Wnt/β-catenin signaling and ZEB1 expression. Mol Med Rep. 2019;19(3):2144–52. https://doi.org/10.3892/mmr.2019.9870.
42. Dávalos A., Goedeke L., Smibert P. et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108(22):9232–7. https://doi.org/10.1073/pnas.1102281108.
43. Pattanayak B., Garrido-Cano I., Adam-Artigues A. et al. MicroRNA-33b suppresses epithelial-mesenchymal transition repressing the MYC-EZH2 pathway in HER2+ breast carcinoma. Front Oncol. 2020;10:1661. https://doi.org/10.3389/fonc.2020.01661.
44. Wu H.T., Zhong H.T., Li G.W. et al. Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer. J Transl Med. 2020;18(1):51. https://doi.org/10.1186/s12967-020-02240-z.
45. Eggers J.C., Martino V., Reinbold R. et al. microRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod Biomed Online. 2016;32(4):434–45. https://doi.org/10.1016/j.rbmo.2015.12.013.
46. Furuya M., Masuda H., Hara K. et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet Gynecol Scand. 2017;96;(9):1128–35. https://doi.org/10.1111/aogs.13179.
47. Zhang Y., Chang X., Wu D. et al. Down-regulation of exosomal miR-214-3p tTargeting CCN2 contributes to endometriosis fibrosis and the role of exosomes in the horizontal transfer of miR-214-3p. Reprod Sci. 2021;28(3):715–27. https://doi.org/10.1007/s43032-020-00350-z.
48. Mani M., Carrasco D.E., Zhang Y. et al. BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res. 2009;69(19):7577–86. https://doi.org/10.1158/0008-5472.CAN-09-0773.
49. Zhang M., Wang X., Xia X. et al. Endometrial epithelial cells-derived exosomes deliver microRNA-30c to block the BCL9/Wnt/CD44 signaling and inhibit cell invasion and migration in ovarian endometriosis. Cell Death Discov. 2022;8(1):151. https://doi.org/10.1038/s41420-022-00941-6.
50. Braza-Boïls A., Marí-Alexandre J., Gilabert J. et al. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29(5):978–88. https://doi.org/10.1093/humrep/deu019.
51. Zhu H., Cao X.X., Liu J., Hua H. MicroRNA-488 inhibits endometrial glandular epithelial cell proliferation, migration, and invasion in endometriosis mice via Wnt by inhibiting FZD7. J Cell Mol Med. 2019;23(4):2419–30. https://doi.org/10.1111/jcmm.14078.
52. Peghaire C., Bats M.L., Sewduth R. et al. Fzd7 (Frizzled-7) Expressed by endothelial cells controls blood vessel formation through Wnt/β-catenin canonical signaling. Arterioscler Thromb Vasc Biol. 2016;36(12):2369–80. https://doi.org/10.1161/ATVBAHA.116.307926.
53. Mai H., Xu H., Lin H. et al. LINC01541 Functions as a ceRNA to modulate the Wnt/β-catenin pathway by decoying miR-506-5p in endometriosis. Reprod Sci. 2021;28(3):665–74. https://doi.org/10.1007/s43032-020-00295-3.
54. Qiao D., Qin X., Yang H. et al. Estradiol mediates the interaction of LINC01541 and miR-429 to promote angiogenesis of G1/G2 endometrioid adenocarcinoma in-vitro: a pilot study. Front Oncol. 2022;12:951573. https://doi.org/10.3389/fonc.2022.951573.
55. Wang Y.Y., Duan H., Wang S. et al. Talin1 induces epithelial-mesenchymal transition to facilitate endometrial cell migration and invasion in adenomyosis under the regulation of microRNA-145-5p. Reprod Sci. 2021;28(5):1523–39. https://doi.org/10.1007/s43032-020-00444-8.
Об авторах
Е. В. ВиривскаяРоссия
Виривская Елена Владимировна - к.м.н.
302026 Орел, Комсомольская ул., д. 95; 127006 Москва, ул. Садовая-Каретная, д. 8, стр. 6
И. В. Игнатко
Россия
Игнатко Ирина Владимировна - д.м.н., проф., член-корр. РАН.
119048 Москва, ул. Трубецкая, д. 8, стр. 2
Scopus Author ID 15118951800, WoS ResearcherID H-2442-2018
И. А. Снимщикова
Россия
Снимщикова Ирина Анатольевна - д.м.н., проф.
302026 Орел, Комсомольская ул., д. 95
К. Р. Бахтияров
Россия
Бахтияров Камиль Рафаэльевич - д.м.н., проф.
119048 Москва, ул. Трубецкая, д. 8, стр. 2
Scopus Author ID 57208396965
А. С. Зуева
Россия
Зуева Алина Сергеевна.
119048 Москва, ул. Трубецкая, д. 8, стр. 2
Scopus Author ID 59132162700, WoS ResearcherID JWO-2945-2024
Т. Д. Капырина
Россия
Капырина Татьяна Дмитриевна.
119048 Москва, ул. Трубецкая, д. 8, стр. 2
Scopus Author ID 58631437000
Что уже известно об этой теме?
► Wnt/β-катениновый сигнальный путь играет ключевую роль в регуляции клеточной пролиферации, миграции и фиброза.
► Гиперактивация Wnt/β-катенинового пути наблюдается при различных опухолевых процессах, включая эндометриоз.
Что нового дает статья?
► Определена роль микроРНК (например, miR-30c, miR-488) в модуляции Wnt/β-катенинового пути при эндометриозе.
► Описаны белки-активаторы, регулирующие Wnt-сигналинг.
Как это может повлиять на клиническую практику в обозримом будущем?
► МикроРНК могут стать новыми биомаркерами для неинвазивной диагностики эндометриоза.
► Таргетная терапия, направленная на Wnt/β-катениновый путь, может улучшить лечение эндометриоза.
Рецензия
Для цитирования:
Виривская Е.В., Игнатко И.В., Снимщикова И.А., Бахтияров К.Р., Зуева А.С., Капырина Т.Д. Роль Wnt/β-катенинового сигнального пути в патогенезе эндометриоза. Акушерство, Гинекология и Репродукция. 2025;19(4):534-544. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2025.640
For citation:
Virivskaya E.V., Ignatko I.V., Snimshchikova I.A., Bakhtiyarov K.R., Zueva A.S., Kapyrina T.D. Wnt/β-catenin signaling pathway role in the endometriosis pathogenesis. Obstetrics, Gynecology and Reproduction. 2025;19(4):534-544. (In Russ.) https://doi.org/10.17749/2313-7347/ob.gyn.rep.2025.640

Контент доступен под лицензией Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































