Scroll to:
The concept of thromboinflammation underlying thrombotic complications, tumor progression and metastasis in gynecological cancer patients
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.542
Abstract
The results of recent studies show that tumor biology, coagulation activation, and inflammatory reactions profoundly contribute to the thrombosis pathogenesis in cancer as well as tumor progression, metastasis, and developing chemoresistance. Cancer is an independent predictor of thrombosis. During carcinogenesis, tumor cells express proinflammatory cytokines, proangiogenic and procoagulant factors, and also stimulate other cells to express various components promoting emerging thromboinflammation. The discovery of neutrophil extracellular traps (NETs) provides an opportunity to take a new look at biology and a role neutrophils may play in thromboinflammation and tumorigenesis. The close interplay between tumor cells, tumor-associated neutrophils and NETs as well as other players in the tumor microenvironment underlies activation of thromboinflammation in cancer patients not only resulting in thrombus formation, but also promoting tumor growth and dissemination.
Keywords
For citations:
Makatsariya A.D., Slukhanchuk E.V., Bitsadze V.O., Solopova A.G., Khizroeva J.Kh., Ashrafyan L.A., Serov V.N., Voynovskiy A.Е., Ungiadze J.Yu., Lazarchuk A.V., Tretyakova M.V., Makatsariya N.A., Salnikova P.V., Gashimova N.R., Grigoreva K.N., Zakashansky K.L., Elalamy I., Gris J. The concept of thromboinflammation underlying thrombotic complications, tumor progression and metastasis in gynecological cancer patients. Obstetrics, Gynecology and Reproduction. 2024;18(4):450-463. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.542
Introduction / Введение
For almost 150 years, there has been known the close connection between cancer and thrombosis. In the last 20 years, the international community focused significant attention on the problem of cancer-associated thrombosis. The relevant mechanisms and biomarkers have been extensively investigated, and new risk assessment scales for cancer-associated thrombosis as well as a search for new therapeutic targets is underway. Besides that thromboembolic complications the cause a direct harm to cancer patients, cancer-associated thrombosis hinders proper antitumor therapy. Patients on long-term anticoagulant therapy after a thrombosis episode often cannot undergo surgical intervention due to high risk, nor can they receive several effective chemotherapy agents with prominent procoagulant effects. Moreover, prolonged use of anticoagulants increases the risk of bleeding in cancer patients. Although oral anticoagulants, Xa inhibitors approved for use in patients with cancer-associated thrombosis, demonstrated effectiveness and the opportunity for use as an alternative means to low molecular weight heparin, they, nevertheless, may result in increased risk of bleeding, especially in certain tumors, and interactions with antitumor agents.
Traditionally, cancer-associated thrombosis has been viewed solely from the perspective of venous thromboembolism. However, recent studies demonstrated that oncological patients also have an increased risk of arterial thrombosis. Currently, cancer-related arterial thrombosis is a promising task for development focused on clarifying the underlying mechanisms and risk factors.
The increased risk of thrombosis observed in gynecological cancer patients has been traditionally explained by cancer potential to affect all components of Virchow's triad (hypercoagulation, stasis, and endothelial dysfunction). Numerous research results obtained in recent years allow us to describe thrombosis pathogenesis in cancer patients by another triad, namely, the thromboinflammation triad (Fig. 1) that will consist of tumor tissue-related biological properties and hemostasis activation as well as tumor-triggered inflammatory reactions. It is now evident that dysregulated hemostasis in cancer patients not only leads to thrombosis but also promotes tumor growth and metastasis spreading.
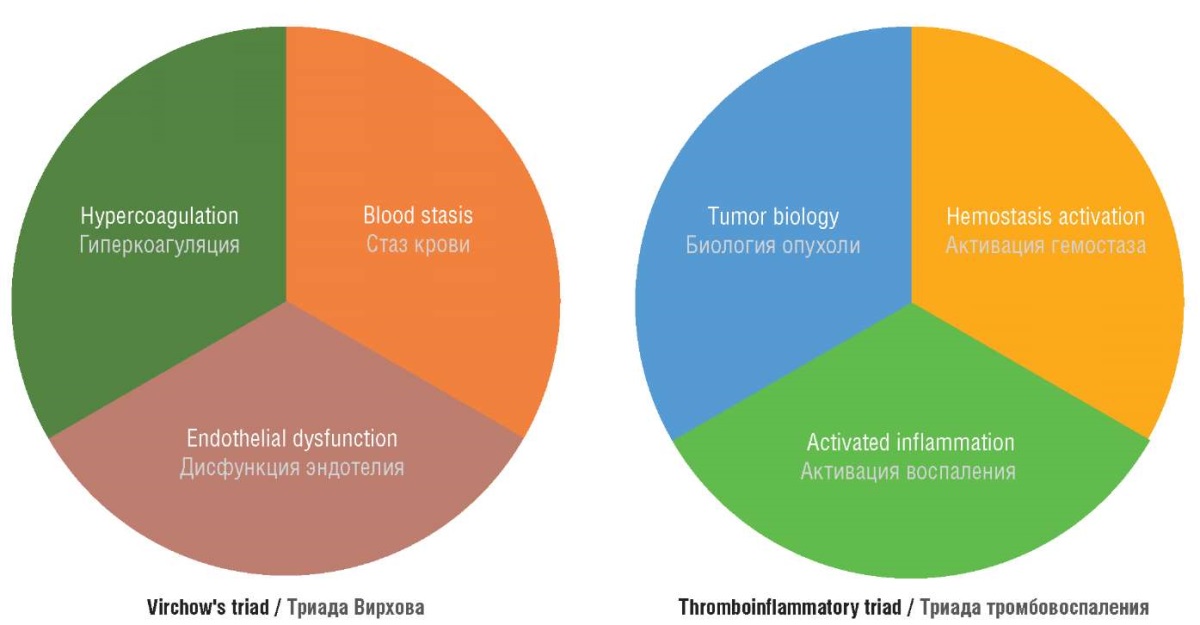
Figure 1. Virchow's triad and thromboinflammation triad [drawn by authors].
Рисунок 1. Триада Вирхова и триада тромбовоспаления [рисунок авторов].
Thromboinflammation: definition of concept / Тромбовоспаление: определение понятия
The concept of thromboinflammation is generally traced back to 2004 when V. Brinkmann and colleagues discovered neutrophil extracellular traps (NETs) and the process by which they are formed, known as NETosis [1]. The concept of thromboinflammation encompasses the mutual activation of the hemostasis system and inflammatory responses [2].
The process of thromboinflammation results from development and interaction between other simpler events in living organisms. In the early stages of animal evolution, responses to injury and infectious agents were unified. Such responses are the prototypes of modern thromboinflammatory reactions. For example, coagulation accounted not only for hemostasis after a blood vessel damage but also for inflammation and regeneration. In some invertebrates, coagulation still occurs in the hemolymph involving hemocytes. In more developed vertebrates, the latter represent predecessors of modern platelets. The external factor’s influence results in initiating hemocyte-mediated hemolymph coagulation along with parallel pathogen capture to limit their spread. This process is an early fundamental response that later divided into three systems: hemostasis, immunity, and inflammation. Today, in humans, thromboinflammation involves platelets, the hemostasis system, complement components, cells participating in inflammatory responses, leukocytes, neutrophils, the pro-inflammatory cytokines they release, complex process as well as NETs as one of the outcomes of multi-faceted events, and innate immune cells. Thromboinflammatory responses were identified in various diseases, including critical conditions such as sepsis and stroke.
Neutrophils / Нейтрофилы
Polymorphonuclear neutrophil is the most common type of leukocytes. The name of this cell type is accounted for by the lack of staining during laboratory diagnostics and observed lobed nuclear structure. Neutrophils are the primary cells of antimicrobial defense due to their powerful arsenal of antimicrobial granules. Tumor is a sort of non-healing wound. Among the many components of tumor microenvironment, neutrophils and their products play a crucial role in tumor progression, immune evasion, and metastasis [3].
Neutrophils destroy pathogens using a combination of mechanisms such as oxidative burst, phagocytosis, release of antimicrobial substances, and NETs. The latter is the major player in thromboinflammation leading to dysregulation of all hemostatic components.
Neutrophils are recruited to the site of inflammation via 3 stages: activation, adhesion, and extravasation mediated by chemokines and selectins [4]. NETosis is induced by various agents and pro-inflammatory mediators, including chemokines, which are abundantly produced in tumor tissue microenvironment. Recent studies demonstrated that neutrophils exhibit neutrophil adaptive (memory-like) responses. For example, the use of BCG (Bacillus Calmette-Guérin) promotes development of adaptive response in native neutrophils [5], leading to the reprogramming of neutrophil transcriptome, epigenetic modifications, and release of pro-inflammatory mediators. Upon repeated insult, neutrophils are rapidly attracted and activated resulting in stronger immune response.
Platelets activate tumor cells, which in turn activate platelets, creating a vicious cycle of cancer-associated thrombosis. Additionally, platelets stimulate neutrophils to release NETs, which intensifies subsequent thromboinflammatory reactions, tumor progression as well as developing metastases [2]. NETs are the key players in thromboinflammation. All types of thrombi in cancer patients contain NETs, indicating that thromboinflammation is an integral part of thrombosis pathogenesis [6].
Neutrophil extracellular traps / Внеклеточные ловушки нейтрофилов
Neutrophils represent the source of extracellular traps formed via a complex cascade of reactions known as NETosis. NETs consist of decondensed DNA strands, proteins, and histones. DNA strands create a network structure wherein the other components of NETs are "trapped." The damaging effects on tissues primarily result from neutrophil elastase (NE), myeloperoxidase (MPO), and cathepsin G contained inside NETs [2][7]. NETosis can vary in intensity, being either physiological or excessive. The latter contributes to pathological thrombosis, hemorrhages, acute inflammation, and tissue destruction [8]. The involvement of NETs in the pathogenesis of various conditions has already been established for autoimmune diseases such as psoriasis, systemic lupus erythematosus, and rheumatoid arthritis, as well as atherosclerosis, vasculitis, cancer, etc. [9].
Proinflammatory cytokines / Провоспалительные цитокины
Interleukin (IL) concentrations are significantly elevated in cancer patients, particularly those with venous thrombosis. Studies demonstrated that interleukins, especially IL-8 in the context of its overexpression in non-small cell lung cancer, directly affect NETosis magnitude [10][11]. Tumor cells actively secrete proinflammatory cytokines. NETs promote cytokine production in macrophages [12]. Cytokines, in turn, participate in NETosis. In vitro studies demonstrated that IL-1β induces NETs formation. In this case, NETosis was not suppressed by the interleukin-1 receptor antagonist (IL-1RA) [13].
Interleukin-8 is secreted by macrophages, endothelial cells, and epithelial cells expressing Toll-like receptors (TLRs) [13]. IL-8 facilitates neutrophil recruitment to the site of inflammation and subsequent NETosis. Malignant cells in various tumors (nasopharyngeal carcinoma, hepatocellular carcinoma, prostate cancer, colorectal cancer) increase IL-8 concentration [14]. The plasma IL-8 level in patients with ovarian tumors is significantly reduced during or after chemotherapy using paclitaxel, suggesting that IL-8 is a promising marker in cancer treatment. IL-8 binds to neutrophil membrane IL-8-R1/2 receptors to govern neutrophils to tumors [15][16]. High levels of circulating IL-8 have been described in cancer patients [17]. IL-8 promotes tumor growth and invasion, the formation of de novo tumor vasculature, and metastatic spread [18]. A more malignant phenotype with a worse prognosis is observed in tumors that produce large amounts of IL-8 [19].
IL-1 and IL-6 activate megakaryopoiesis and increase platelet level. IL-2 reduces the secretion of platelet alpha granules, whereas interferon gamma (IFN-γ) and IL-1 enhance the release of dense granules. The thrombomodulin–protein C–protein S pathway is suppressed by IL-1 and tumor necrosis factor alpha (TNF-α). Endothelial cells and monocytes release large amounts of tissue factor (TF) in response to TNF-α and IL-6 [20][21]. IL-1, TNF-α, and IFN-γ trigger endothelial cells to release plasminogen activator inhibitor-1 (PAI-1) [1][22].
Structural components of neutrophil extracellular traps / Структурные компоненты внеклеточных ловушек нейтрофилов
Thromboinflammation transits from a normal response to a pathological process upon excessive NETosis resulting either from enhanced NETs synthesis or its failed clearance. During inflammation, circulating exogenous and endogenous DNases break down NETs followed by release of histone-proteases associated with DNA to unveil their proteolytic properties. Such histone-proteases degrade the extracellular matrix, damage the endothelium, and harm other cells [23]. After histone-caused endothelial damage, the endothelium begins to release Н2О2, which repeatedly triggers NETosis.
NETs influence all components of the hemostatic system [24]. Hemostasis is activated through both intrinsic and extrinsic pathways. NETs-contained DNA, along with TF, acts as a cofactor in thrombin-dependent activation of factor XI, participating in activation of extrinsic pathway [25]. NETs DNA also facilitates activation of intrinsic pathway on negatively charged surfaces by activating factor XII [26]. NETs histones activate platelets in conjunction with thrombin [27]. Histone H4, by binding to prothrombin, triggers its activation.
NETs components hinder fibrinolysis through several mechanisms. The stabilization of fibrin protofibrils occurs through histone-related lateral aggregation. Both non-covalent and covalent bonds with histones further enhance fibrin thickening. Plasmin is unable to fully carry out fibrinolysis due to NETs DNA integration into the fibrin matrix. Histones act as targets that inhibit plasmin activity by occupying plasminogen fibrin-binding sites [6][28]. Additionally, NETs DNA disrupts tPA-mediated (tissue plasminogen activator, tPA) plasminogen-to-plasmin conversion by forming PAI-1 and tPA complexes [29].
NETs via histones alter function of key anticoagulants. Activated protein C (APC) is inactivated by neutrophil oxidase and elastase. Histones counter interaction between antithrombin, thrombomodulin, and thrombin [30].
Endothelial activation triggered partially by NETs components leads to von Willebrand factor (vWF) release. Upon exocytosis, vWF attracts a great number of platelets to the site of endothelial damage promoting microthrombus formation. Consequently, NETs interfere with the normal functioning of the ADAMTS-13/vWF axis.
Thus, excessive NETosis in various conditions including cancer patients leads to complete hemostasis dysregulation.
Antiphospholipid antibodies / Антифосфолипидные антитела
Increasing number of studies demonstrate the link between circulating antiphospholipid antibodies (aPL) and thromboinflammatory processes. S. Yalavarthi et al. described NETosis induced by anticardiolipin antibodies (aCL) as a novel thrombosis mechanism in antiphospholipid syndrome (APS) [31]. Supporting the hypothesis that aPL activate neutrophils for subsequent NETosis, it was demonstrated that neutrophils isolated from APS patients enhanced spontaneous NETs release. Additionally, a positive correlation was found between lupus anticoagulant (LA), anti-β2-glycoprotein 1 IgG antibodies (aβ2-GP1), IgG aCL and circulating MPO-DNA complexes in vivo. Using various laboratory methods, β2-GP1 was detected on neutrophil surface underlies that aβ2-GPI binds to neutrophils to trigger NETosis [6].
Molecules released during NETosis can be recognized by the immune system as autoantigens. This creates a vicious cycle of autoimmune reactions, leading to further antigen release [32]. NETosis per se contributes to developing thrombotic events and promotes further aPL production. These two events, mutually reinforcing each other, contribute to emerging prothrombotic state in cancer patients.
Von Willebrand factor and ADAMTS-13 metalloproteinase / Фактор фон Виллебранда и металлопротеиназа ADAMTS-13
The regulation of microthrombus formation in human involves the large multimeric glycoprotein vWF released by the endothelium upon injury. The primary function of this multimer is to recruit, activate and aggregate platelets on the collagen matrix in the subendothelial layer leading to thrombus formation and bleeding cessation. Additionally, vWF is involved in inflammatory responses, tumor growth, angiogenesis, and metastasis; vWF level is markedly elevated in various malignant neoplasms (Fig. 2).
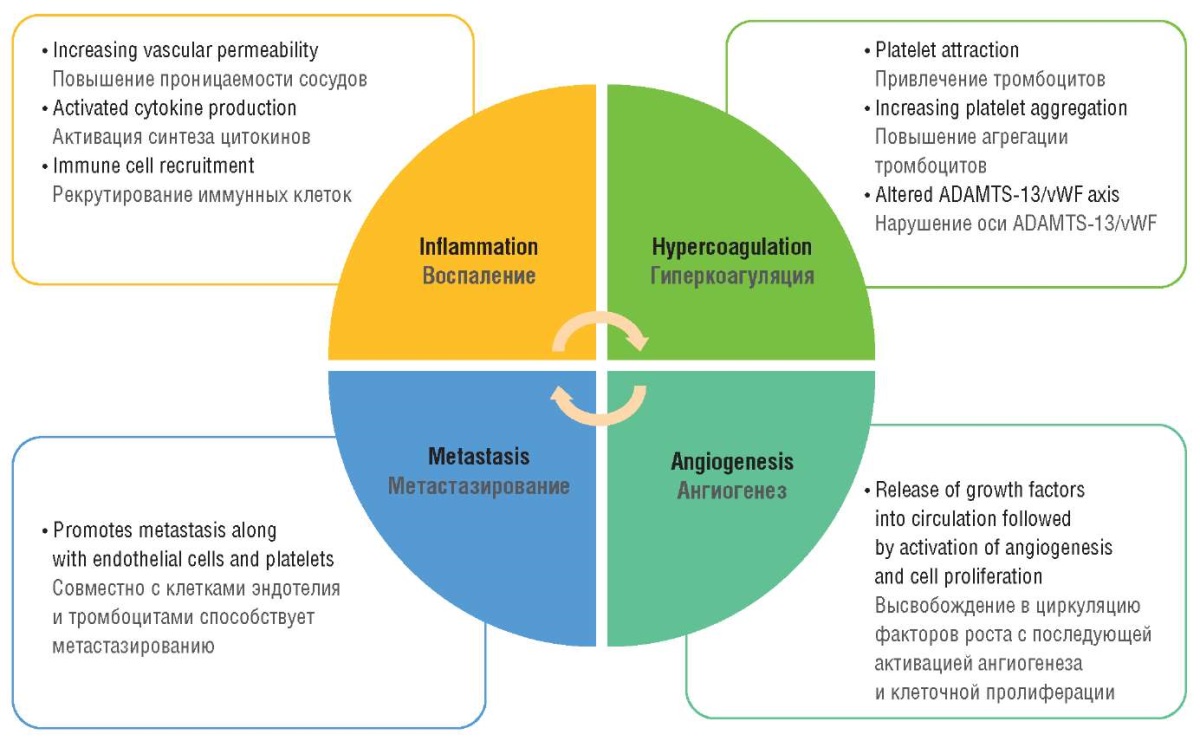
Figure 2. Von Willebrand factor in the pattern of thromboinflammation concept in cancer
[drawn by authors].
Рисунок 2. Фактор фон Виллебранда в структуре концепции тромбовоспаления при раке
[рисунок авторов].
The metalloproteinase ADAMTS-13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) is the key factor regulating vWF multimer quantity. vWF accumulation and decline in ADAMTS-13 levels lead to emergence of vWF-platelet aggregates and fibrin deposition in the microvascular network, the phenomenon known as cancer-associated microangiopathy network. This process is known as the phenomenon of cancer-associated microangiopathy [33].
According to the data, increased vWF concentrations and/or decreased ADAMTS-13 levels have been associated with poorer survival outcomes in colorectal cancer [34], head and neck tumors [35], lung cancer [36], and Waldenström's macroglobulinemia [37]. The mechanism leading to decreased plasma ADAMTS-13 levels in cancer patients is not fully understood. Various oncogenes regulate expression of extracellular proteases, which may directly impair enzyme function.
Congenital and acquired ADAMTS-13 deficiency as well as a decrease in vWF level and activity are potential markers of microthrombosis risk. During growth and invasion, tumor cells disrupt the integrity and activate endothelial cells leading to release of high amounts of vWF multimers and triggers ADAMTS-13 activation. During massive endothelial activation, ADAMTS-13 is actively consumed followed by a decrease in both its level and activity. A direct correlation between the degree of endothelial activation and tumor growth exists. While tumor tissue grows, the magnitude of ADAMTS-13 consumption elevates. A similar scenario occurs in other conditions accompanied by endothelial dysfunction such as systemic inflammatory diseases, sepsis, and disseminated intravascular coagulation (DIC) syndrome. vWF multimers during absolute or relative (in the presence of circulating inhibitors) ADAMTS-13 deficiency trigger platelet activation and aggregation, leading to the formation of mixed tumor-platelet thromboemboli.
Von Willebrand factor facilitates adhesion of tumor cells to the endothelium and their transit through blood vessel wall, thereby promoting metastasis [36]. In in vitro studies, melanoma cells intrinsically activated the endothelium and stimulated vWF multimer release leading to subsequent platelet aggregation and thrombosis [38]. Studies demonstrated that in cancer, a decrease in ADAMTS-13 concentration with excessive vWF release leads to thrombosis. This situation can potentially be corrected by using recombinant ADAMTS-13 (rADAMTS-13) [38].
ADAMTS-13 activity is reduced by NETs, which bind to vWF through electrostatic interactions between free DNA and the A1 domain recruiting more neutrophils to the site [6][39][40], that may be prevented by heparin [41]. Apart from this, NETs DNA occupies glycoprotein Iba (GPIba) binding sites in vWF A1 domain [20][42].
Positively charged NE fragments may result in binding to vWF, a key mediator in recruiting platelets and leukocytes to the endothelium. The interaction with vWF recruits more leukocytes to the activated endothelium [20]. Experiments demonstrated that activated platelets stimulate NETosis only in the presence of vWF [43][44].
A decrease in ADAMTS-13 concentration and activity as well as elevated vWF level and activity are universal microthrombosis mechanisms in sepsis and DIC syndrome [45]. NETs affect the activity of ADAMTS-13 and vWF. Activated neutrophils and NETs via factors such as proteases, peptides, cytokines, and reactive oxygen species modify the structure and conformation of ADAMTS-13 binding sites.
Peptidyl arginine deiminase 4 (PAD4) participates in NETosis and protein citrullination. PAD4 is found in leukocyte nucleus. Citrullination ensures conversion of protein arginine residues into citrulline residues acted upon by PAD4, which removes protein charge [46–49]. After neutrophils become recruited and stimulated, NADPH oxidase is phosphorylated, reactive oxygen species are synthesized, and histones undergo citrullination. PAD4 inhibition lowers NETosis magnitude by preventing histone H3 citrullination and subsequent NETs synthesis [50]. Mice lacking PAD4 are unable to undergo chromatin decondensation and subsequent NETosis [49][51].
Peptidyl arginine deiminase 4 is also an integral NETs component that participates in NETs formation by converting arginine residues in histones to citrulline [52] and decondensing chromatin. PAD4 is able to citrullinate ADAMTS-13 in plasma by modifying arginine residues and, thereby altering its structure as well as activity [20][53].
NETs contain alpha-defensins, also known as human neutrophil peptides (HNPs), which participate in immune responses in vivo. Due to their ability to activate platelets [54] and reduce fibrinolysis, they exhibit procoagulant properties [55]. HNPs bind to vWF A2 domain, thereby modulating the ADAMTS-13/vWF axis. Studies demonstrated elevated plasma HNPs levels in patients with acute thrombotic thrombocytopenic purpura (TTP) [20][56].
One of NETs proteases is myeloperoxidase, which catalyzes the synthesis of hypochlorous acid from H2O2 and Cl–. Hypochlorous acid oxidizes methionine to methionine sulfoxide. This occurs at ADAMTS-13 cleavage site within vWF A2 domain and in ADAMTS-13 by affecting function of the ADAMTS-13/vWF axis [57–59]. MPO–H2O2–Cl– system during NETosis may result in imbalanced ADAMTS-13/vWF axis and microvascular thrombosis [20]. Both plasmin and NE cleave ADAMTS-13 in plasma in vitro. Research has demonstrated the contribution of NETosis and thromboinflammation to extensive microthrombosis in patients with acute forms of TTP, who exhibit elevated plasma concentrations of NETs components, including DNA-histone complexes and MPO, along with reduced platelet counts [20][60].
Pro-inflammatory cytokines regulate the release of endothelial vWF multimers and their cleavage into smaller fragments [20][61]. IL-6 inhibits cleavage of vWF multimers by the ADAMTS-13. The IL-6/IL-6 receptor complex, TNF-α, and IL-8 enhance release of vWF multimers [62].
In DIC syndrome associated with sepsis, low-molecular-weight ADAMTS-13 isoforms are detected [56]. The decrease in ADAMTS-13 level and activity during excessive NETosis facilitates circulation of vWF multimers, which recruit and activate more neutrophils and platelets. Administering DNase I or recombinant ADAMTS-13 may provide a potential option to break this vicious cycle [20].
Thromboinflammation and chemotherapy resistance / Тромбовоспаление и резистентность к химиотерапии
Tumor tissue has long been considered the primary source of circulating plasma free DNA (cfDNA) in cancer patients. During tumor progression, cfDNA resembles DNA from NETs, suggesting that NETosis may underlie chemoresistance [63].
Experiments with PAD4+/+ mice bearing lung tumors using platinum-based chemotherapeutic agents were associated with cfDNA release and thrombus formation not observed in PAD4–/– mice, suggesting that an increase in cfDNA and thrombin levels during platinum-based chemotherapy is directly related to PAD4 and NETosis confirmed by other studies (Fig. 3) [49][64][65]. It is NETosis, rather than apoptosis or necrosis that promotes blood plasma cfDNA levels [65].
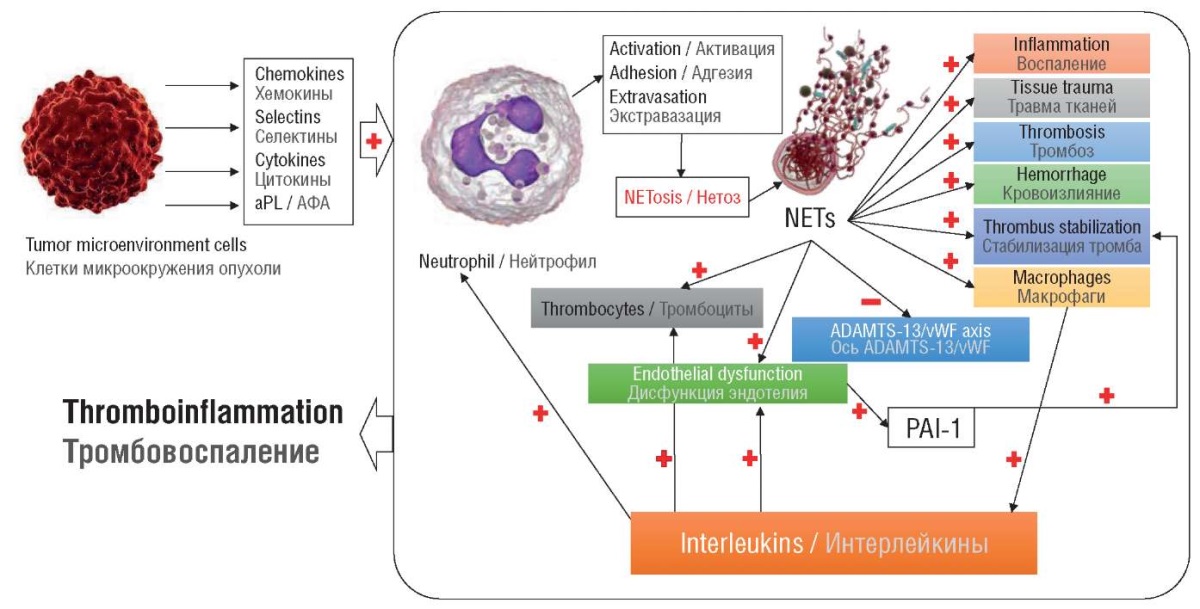
Figure 3. Integral concept of thromboinflammation in cancer [drawn by authors].
Note: aPL – antiphospholipid antibodies; NETs – neutrophil extracellular traps;
vWF – von Willebrand factor; PAI-1 – plasminogen activator inhibitor-1.
Рисунок 3. Интегральная концепция тромбовоспаления при раке [рисунок авторов].
Примечание: АФА – антифосфолипидные антитела;
NETs – внеклеточные ловушки нейтрофилов; vWF – фактор фон Виллебранда;
PAI-1 – ингибитор активатора плазминогена-1.
In PAD4+/+ mice undergoing chemotherapy, DNase administration reduced the risk of thrombus formation, an effect not observed in PAD4–/– mice [66]. Tumor-associated neutrophils, acted upon by granulocyte colony-stimulating factor (G-CSF) trigger NETosis [64]. Hence, the results of such studies establish a link between tumor progression, neutrophil count, and increased blood plasma levels of G-CSF and cfDNA in cancer patients [66].
Neutrophils have long been associated with poor response to immune checkpoint inhibitor therapies. Recent studies demonstrated that NETosis indeed underlies the poor response to chemotherapy [67]. In the case of immune checkpoint inhibitor therapy for pancreatic adenocarcinoma, NETosis shields tumor cells from CD8+ T-cells action [49][68].
By enveloping the tumor cell and preventing contact with CD8+ T-cells and natural killer cells, NETs mechanically protect the tumor in vitro. This protective barrier can be disrupted in experiments by administering DNase 1. However, supraphysiological DNase 1 concentrations are required for NETs degradation [49][69][70]. Additionally, the effectiveness of thrombolysis is enhanced when blood clots are simultaneously treated with DNase and tPA [49][71].
The role of NE, matrix metalloproteinase (MMP) MMP-9, cathepsin G, programmed cell death ligand 1 (PDL-1), and carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1) in emerging chemoresistance has now been established [49][72]. MMP-9 is a metalloproteinase that degrades the extracellular matrix (ECM) [73]. Studies demonstrated tumor chemoresistance and ECM degradation associated with elevated MMP-9 expression in gastric cancer [74]. MMP-9 also contributes to tumor neoangiogenesis and reduces intra-tumoral perfusion of chemotherapeutic agents [49][75]. One of NETs components, NE, promotes tumor growth by influencing the epithelial-mesenchymal transition, which transforms the tumor cell into a mesenchymal phenotype [72][76]. Compared to rental cells, mesenchymal phenotype cells have a greater capacity for migration and apoptosis [49][77]. The transmembrane glycoprotein CEACAM1 also found in NETs takes part in T-cell pool depletion and activation of tumor cell adhesion and migration [49][78]. The programmed cell death protein 1 (PD-1) membrane receptor normally regulates T-cell antitumor activity. T-cell depletion occurs through the interaction between PD-1 and PDL-1 (NETs component) which inevitably leads to arising resistance to immunotherapy [79–81]. The involvement of neutrophils and NETs in formation of chemoresistance is multifaceted and requires further investigation, as do the potential pathways to overcome this resistance.
The link between neutropenia and improved survival prognosis has long attracted attention but lacked a logical explanation. This phenomenon can now be accounted for by understanding the mechanisms of thromboinflammation. In this context, neutropenia not only appears to be a marker of effective chemotherapy but also confirms the existence of NETs-dependent chemoresistance mechanisms.
Conclusion / Заключение
Overall, thromboinflammation per se and NETosis in particular are specific mechanisms whose significance in clinical oncology is only now being explored. Both clinical and basic research data presented here confirm a need for further investigation of thromboinflammation. The relevance of thromboinflammation and hemostasis disorders in cancer patients is driven by the necessity to improve the principles of complication prevention and predictionof disease progression. The molecular mechanisms of cancer-associated thrombosis, tumor progression, and metastasis in gynecologic oncology patients, taking into account new findings on thromboinflammation in oncogynecology as well as laboratory diagnostics and therapy remain highly relevant issues for modern medicine.
References
1. Brinkmann V., Reichard U., Goosmann C. et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-5. https://doi.org/10.1126/science.1092385.
2. BitsadzeV.O., Slukhanchuk E.V., Solopova A.G. et al. The role of the microenvironment in tumor growth and spreading. [Rol' mikrookruzheniya v roste i rasprostranenii opuholi]. Obstetrics, Gynecology and Reproduction. 2024;18(1):96—111. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.489.
3. Slukhanchuk E.V., Bitsadze V.O., Solopova A.G. et al. Thromboinflammation in oncogynecological patients. [Trombovospalenie u onkologicheskih bol'nyh]. Obstetrics, Gynecology and Reproduction. 2022;16(5):611-22. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.355.
4. Leal A.C., Mizurini D.M., Gomes T. et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. SciRep. 2017;7(1):1—12. https://doi.org/10.1038/s41598-017-06893-7.
5. Moorlag S.J., Rodriguez-Rosales Y.A., Gillard J. et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 2020;33(7):108387. https://doi.org/10.1016/j.celrep.2020.108387.
6. Makatsariya A.D., Slukhanchuk E.V., Bitsadze V.O. et al. Thrombotic storm, hemostasis disorders and thromboinflammation in COVID-19. [Tromboticheskij shtorm, narusheniya gemostaza i trombovospalenie v usloviyah COVID-19]. Obstetrics, Gynecology and Reproduction. 2021;15(5):499-514. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2021.247.
7. Slukhanchuk E.V., Bitsadze V.O., Solopova A.G. et al. An impact of neutrophil extracellular traps to the prothrombotic state and tumor progression in gynecological cancer patients. [Vklad vnekletochnyh lovushek nejtrofilov v protromboticheskoe sostoyanie i progressiyu opuholi u onkoginekologicheskih pacientok]. Obstetrics, Gynecology and Reproduction. 2023;17(1):53-64. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.385.
8. Slukhanchuk E.V., Bitsadze V.O., Solopova A.G. et al. Neutrophil extracellular traps as markers of thromboinflammation in the pathogenesis of female genital tract and breast malignant neoplasms. [Vnekletochnye lovushki nejtrofilov kak markery trombovospaleniya v patogeneze zlokachestvennyh novoobrazovanij zhenskih polovyh organov i molochnoj zhelezy]. Obstetrics, Gynecology and Reproduction. 2022;16(4):426-37. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.335.
9. Slukhanchuk E.V. NETs and oncologic process. [NETs i onkologicheskij process]. Obstetrics, Gynecology and Reproduction. 2021;15(1):107-16. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2021.204.
10. Sunaga N., Imai H., Shimizu K. et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int J Cancer. 2012;130(8):1733-44. https://doi.org/10.1002/ijc.26164.
11. Alfaro C., Teijeira A., Onate C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res. 2016;22(15):3924— 36. https://doi.org/10.1158/1078-0432.CCR-15-2463.
12. Magna M., Pisetsky D.S. The alarmin properties of DNA and DNA-associated nuclear proteins. Clin Ther. 2016;38(5):1029-41. https://doi.org/10.1016/j.clinthera.2016.02.029.
13. Meher A.K., Spinosa M., Davis J.P. et al. Novel role of IL (interleukin)-1 p in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38(4):843-53. https://doi.org/10.1161/ATVBAHA.117.309897.
14. Zarogoulidis P., Katsikogianni F., Tsiouda T. et al. Interleukin-8 and interleukin-17 for cancer. Cancer Invest. 2014;32(5):197-205. https://doi.org/10.3109/07357907.2014.898156.
15. Kohidai L., Csaba G. Chemotaxis and chemotactic selection induced with cytokines (IL-8, Rantes and TNF-alpha) in the unicellular Tetrahymena pyriformis. Cytokine. 1998;10(7):481-6. https://doi.org/10.1006/cyto.1997.0328.
16. Nie M., Yang L., Bi X. et al. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling NETs promote DLBCL progression. Clin Cancer Res. 2019;25(6):1867-79. https://doi.org/10.1158/1078-0432.CCR-18-1226.
17. Baggiolini M., Walz A., Kunkel S. Neutrophil-activating peptide-1/ interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84(4):1045-9. https://doi.org/10.1172/JCI114265.
18. Xu H., Zeng Y., Liu L. et al. PRL-3 improves colorectal cancer cell proliferation and invasion through IL-8 mediated glycolysis metabolism. Int J Oncol. 2017;51(4):1271-9. https://doi.org/10.3892/ijo.2017.4090.
19. Sanmamed M.F., Carranza-Rua O., Alfaro C. et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins serum IL8 and tumor burden. Clin Cancer Res. 2014;20(22):5697-707. https://doi.org/10.1158/1078-0432.CCR-13-3203.
20. Slukhanchuk E.V., Bitsadze V.O., Solopova A.G. et al. Immunothrombosis in cancer patients: contribution of neutrophil extracellular traps, ADAMTS-13 and von Willebrand factor. [Immunotromboz u onkologicheskih bol'nyh: vklad vnekletochnyh lovushek nejtrofilov, ADAMTS-13 i faktora fon Villebranda]. Obstetrics, Gynecology and Reproduction. 2022;16(6):648-63. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.364.
21. Iupatov E.Iu., Mustafin I.G., Kurmanbaev T.E. et al. Local hemostasis disorders underlying endometric pathology. [Rol' narushenij lokal'nogo gemostaza v geneze patologii endometriya]. Obstetrics, Gynecology and Reproduction. 2021;15(4):430-40. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2021.214.
22. Malte A.L., H0jbjerg J.A., Larsen J.B. Platelet parameters as biomarkers for thrombosis risk in cancer: A systematic review and meta-analysis. Semin Thromb Hemost. 2024;50(3):360-83. https://doi.org/10.1055/s-0043-1764381.
23. Jiménez-Alcázar M., Napirei M., Panda R. et al. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost. 2015;13(5):732-42. https://doi.org/10.1111/jth.12796.
24. Gould T., Lysov Z., Liaw P. Extracellular DNA and histones: double-edged swords in immunothrombosis. J Thromb Haemost. 2015;13:S82-S91. https://doi.org/10.1111/jth.12977.
25. Vu T.T., Leslie B.A., Stafford A.R. et al. Histidine-rich glycoprotein binds DNA and RNA and attenuates their capacity to activate the intrinsic coagulation pathway. J Thromb Haemost. 2016;115(01):89-98. https://doi.org/10.1160/TH15-04-0336.
26. Naudin C., Burillo E., Blankenberg S. et al. Factor XII contact activation. Semin Thromb Hemost. 2017;43(8):814-26. https://doi.org/10.1055/s-0036-1598003.
27. Noubouossie D.F., Whelihan M.F., Yu Y.-B. et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129(8):1021-9. https://doi.org/10.1182/blood-2016-06-722298.
28. Longstaff C., Varju I., Sotonyi P. et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288(10):6946-56. https://doi.org/10.1074/jbc.M112.404301.
29. Komissarov A.A., Florova G., Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem. 2011;286(49):41949-62. https://doi.org/10.1074/jbc.M111.301218.
30. Varjú I., Longstaff C., Szabó L. et al. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb Haemost. 2015;113(06):1289-98. https://doi.org/10.1160/TH14-08-0669.
31. Yalavarthi S., Gould T.J., Rao A.N. et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67(11):2990-3003. https://doi.org/10.1002/art.39247.
32. Obermeier H.L., Riedl J., Ay C. et al. The role of ADAMTS-13 and von Willebrand factor in cancer patients: results from the Vienna cancer and thrombosis study. Res Pract Thromb Haemost. 2019;3(3):e12197. https://doi.org/10.1002/rth2.12197.
33. Mazetto B.M., Orsi F.L., Barnabé A. et al. Increased ADAMTS13 activity in patients with venous thromboembolism. Thromb Res. 2012;130(6):889-93. https://doi.org/10.1016/j.thromres.2012.09.009.
34. Liu Y., Starr M.D., Bulusu A. et al. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med. 2013;2(2):234-42. https://doi.org/10.1002/cam4.71.
35. Sweeney J.D., Killion K.M., Pruet C.F., Spaulding M.B. von Willebrand factor in head and neck cancer. Cancer. 1990;66(11):2387-9. https://doi.org/10.1002/1097-0142(19901201)66:11<2387::aid-cncr2820661123>3.0.co;2-u.
36. Guo R., Yang J., Liu X. et al. Increased von Willebrand factor over decreased ADAMTS-13 activity is associated with poor prognosis in patients with advanced non-small-cell lung cancer. J Clin Lab Anal. 2018;32(1):e22219. https://doi.org/10.1002/jcla.22219.
37. Hivert B., Caron C., Petit S. et al. Clinical and prognostic implications of low or high level of von Willebrand factor in patients with Waldenstrom macroglobulinemia. Blood. 2012;120(16):3214—21. https://doi.org/10.1182/blood-2011-11-388256.
38. Bauer A.T., Suckau J., Frank K. et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood. 2015;125(20):3153-63. https://doi.org/10.1182/blood-2014-08-595686.
39. Goertz L., Schneider S.W., Desch A. et al. Heparins that block VEGF-A-mediated von Willebrand factor fiber generation are potent inhibitors of hematogenous but not lymphatic metastasis. Oncotarget. 2016;7(42):68527. https://doi.org/10.18632/oncotarget.11832.
40. Makatsariya A.D., Slukhanchuk E.V., Bitsadze V.O. et al. Neutrophil extracellular traps: a role in inflammation and dysregulated hemostasis as well as in patients with COVID-19 and severe obstetric pathology. [Vnekletochnye lovushki nejtrofilov: uchastie v processah vospaleniya i dizregulyacii gemostaza, v tom chisle u pacientov s COVID-19 i tyazheloj akusherskoj patologiej]. Obstetrics, Gynecology and Reproduction. 2021;15(4):335-50. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2021.238.
41. Huang R.H., Fremont D.H., Diener J.L. et al. A structural explanation for the antithrombotic activity of ARC1172, a DNA aptamer that binds von Willebrand factor domain A1. Structure. 2009;17(11):1476—84. https://doi.org/10.1016/j.str.2009.09.011.
42. Grassle S., Huck V., Pappelbaum K.I. et al. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol. 2014;34(7):1382-9. https://doi.org/10.1161/ATVBAHA.113.303016.
43. Carestia A., Kaufman T., Rivadeneyra L. et al. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leucoc Biol. 2016;99(1):153— 62. https://doi.org/10.1189/jlb.3A0415-161R.
44. Honda M., Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenter Hepatol. 2018;15(4):206-21. https://doi.org/10.1038/nrgastro.2017.183.
45. Farkas P., Csuka D., Mikes B. et al. Complement activation, inflammation and relative ADAMTS13 deficiency in secondary thrombotic microangiopathies. Immunobiology. 2017;222(2):119-27. https://doi.org/10.1016/j.imbio.2016.10.014.
46. Vossenaar E.R., Zendman A.J., van Venrooij W.J., Pruijn G.J. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25(11):1106-18. https://doi.org/10.1002/bies.10357.
47. Hensen S.M., Pruijn G.J. Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination. Mol Cell Proteomics. 2014;13(2):388-96. https://doi.org/10.1074/mcp.R113.033746.
48. van Beers J.J., Zendman A.J., Raijmakers R. et al. Peptidylarginine deiminase expression and activity in PAD2 knock-out and PAD4-low mice. Biochimie. 2013;95(2):299-308. https://doi.org/10.1016/j.biochi.2012.09.029.
49. Slukhanchuk E.V., Bitsadze V.O., Solopova A.G. et al. Neutrophil extracellular traps-associated markers in malignant neoplasms of the female reproductive system after surgical treatment and adjuvant chemotherapy. [Markery vnekletochnyh lovushek nejtrofilov u zhenshchin so zlokachestvennymi novoobrazovaniyami reproduktivnoj sistemy, poluchavshih hirurgicheskoe lechenie i ad"yuvantnuyu himioterapiyu]. Obstetrics, Gynecology and Reproduction. 2023;17(4):420-32. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.432.
50. Lewis H.D., Liddle J., Coote J.E. et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11(3):189-91. https://doi.org/10.1038/nchembio.1735.
51. Martinod K., Demers M., Fuchs T.A. et al. Neutrophil histone modification by peptidyl arginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110(21):8674-9. https://doi.org/10.1073/pnas.1301059110.
52. Hisada Y., Sachetto A.T.A., Mackman N. Circulating tissue factor-positive extracellular vesicles and their association with thrombosis in different diseases. Immunol Rev. 2022;312(1):61-75. https://doi.org/10.1111/imr.13106.
53. Sorvillo N., Mizurini D.M., Coxon C. et al. Plasma peptidylarginine deiminase IV promotes VWF-platelet string formation and accelerates thrombosis after vessel injury. Circ Res. 2019;125(5):507-19. https://doi.org/10.1161/CIRCRESAHA.118.314571.
54. Quinn K., Henriques M., Parker T. et al. Human neutrophil peptides: a novel potential mediator of inflammatory cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2008;295(5):H1817-24. https://doi.org/10.1152/ajpheart.00472.2008.
55. Higazi A.A., Ganz T., Kariko K., Cines D.B. Defensin modulates tissue-type plasminogen activator and plasminogen binding to fibrin and endothelial cells. J Biol Chem. 1996;271(30):17650-5. https://doi.org/10.1074/jbc.271.30.17650.
56. Pillai V.G., Bao J., Zander C.B. et al. Human neutrophil peptides inhibit cleavage of von Willebrand factor by ADAMTS13: a potential link of inflammation to TTP. Blood. 2016;128(1):110-9. https://doi.org/10.1182/blood-2015-12-688747.
57. Crawley J.T., Lam J.K., Rance J.B. et al. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105(3):1085-93. https://doi.org/10.1182/blood-2004-03-1101.
58. Chen J., Fu X., Wang Y. et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115(3):706-12. https://doi.org/10.1182/blood-2009-03-213967.
59. Nishimura K., Sano M., Ohtaka M. et al. Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011;286(6):4760-71. https://doi.org/10.1074/jbc.M110.183780.
60. Zheng L., Abdelgawwad M.S., Zhang D. et al. Histone-induced thrombotic thrombocytopenic purpura in adamts13-/-zebrafish depends on von Willebrand factor. Haematologica. 2020;105(4):1107-19. https://doi.org/10.3324/haematol.2019.237396.
61. Richardson P.G., Chanan-Khan A., Schlossman R.L. et al. Phase II trial of Single Agent Bortezomib (VELCADE®) in patients with previously untreated multiple myeloma (MM). Blood. 2004;106(11):336. https://doi.org/10.1182/blood.V104.11.336.336.
62. Ono T., Mimuro J., Madoiwa S. et al. Severe secondary deficiency of von Willebrand factor - cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107(2):528-34. https://doi.org/10.1182/blood-2005-03-1087.
63. Lysov Z., Dwivedi D.J., Gould T.J., Liaw P.C. Procoagulant effects of lung cancer chemotherapy: impact on microparticles and cell-free DNA. Blood Coagul Fibrinolysis. 2017;28(1):72-82. https://doi.org/10.1097/MBC.0000000000000546.
64. Swystun L.L., Mukherjee S., Liaw P.C. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9(11):2313-21. https://doi.org/10.1111/j.1538-7836.2011.04465.x.
65. Lysov Z., Swystun L.L., Kuruvilla S. et al. Lung cancer chemotherapy agents increase procoagulant activity via protein disulfide isomerasedependent tissue factor decryption. Blood Coagul Fibrinolysis. 2015;26(1):36-45. https://doi.org/10.1097/MBC.0000000000000145.
66. Demers M., Krause D.S., Schatzberg D. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076-81. https://doi.org/10.1073/pnas.1200419109.
67. Zhang Y., Guoqiang L., Sun M., Lu X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med. 2020;17(1):32-43. https://doi.org/10.20892/j.issn.2095-3941.2019.0372.
68. Zhang Y., Chandra V., Riquelme Sanchez E. et al. Interleukin-17—induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217(12):e20190354. https://doi.org/10.1084/jem.20190354.
69. Farrera C., Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191(5):2647-56. https://doi.org/10.4049/jimmunol.1300436.
70. Teijeira A., Garasa S., Gato M. et al. Cxcrl and cxcr2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 19;52(5):856—71.e8. https://doi.org/10.1016/j.immuni.2020.03.001.
71. Fuchs T.A., Brill A., Duerschmied D. et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880—5. https://doi.org/10.1073/pnas.1005743107.
72. Cools-Lartigue J., Spicer J., Najmeh S., Ferri L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci. 2014;71:4179-94. https://doi.org/10.1007/s00018-014-1683-3.
73. Cabral-Pacheco G.A., Garza-Veloz I., Castruita-De la Rosa C. et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739. https://doi.org/10.3390/ijms21249739.
74. Gao H., Lan X., Li S., Xue Y. Relationships of MMP-9, E-cadherin, and VEGF expression with clinicopathological features and response to chemosensitivity in gastric cancer. Tumor Biol. 2017;39(5):1010428317698368. https://doi.org/10.1177/1010428317698368.
75. Viallard C., Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409-26. https://doi.org/10.1007/s10456-017-9562-9.
76. Zhu T., Zou X., Yang C. et al. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelial-mesenchymal transition. Int J Mol Med. 2021;48(1):1-13. https://doi.org/10.3892/ijmm.2021.4960.
77. Ribatti D., Tamma R., Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. 2020;13(6):100773. https://doi.org/10.1016/j.tranon.2020.100773.
78. Rayes R.F., Vourtzoumis P., Rjeily M.B. et al. Neutrophil extracellular trap- associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol. 2020;204(8):2285-94. https://doi.org/10.4049/jimmunol.1900240.
79. Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12(6):492-9. https://doi.org/10.1038/ni.2035.
80. Jiang W., He Y., He W. et al. Exhausted CD8+ T cells in the tumor immune microenvironment: new pathways to therapy. Front Immunol. 2021;11:622509. https://doi.org/10.3389/fimmu.2020.622509.
81. Kaltenmeier C., Yazdani H.O., Morder K. et al. Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front Immunol. 2021;12:785222. https://doi.org/10.3389/fimmu.2021.785222.
About the Authors
A. D. MakatsariyaRussian Federation
Alexander D. Makatsariya - MD, Dr Sci Med, Prof., Academician of RAS.
8 bldg. 2, Trubetskaya Str., Moscow 119991
Scopus Author ID 57222220144; WoS ResearcherID M-5660-2016
E. V. Slukhanchuk
Russian Federation
Ekaterina V. Slukhanchuk - MD, PhD.
8 bldg. 2, Trubetskaya Str., Moscow 119991
V. O. Bitsadze
Russian Federation
Victoria O. Bitsadze - MD, Dr Sci Med, Prof., Professor of RAS.
8 bldg. 2, Trubetskaya Str., Moscow 119991
Scopus Author ID 6506003478; WoS ResearcherID F-8409-2017
A. G. Solopova
Russian Federation
Antonina G. Solopova - MD, Dr Sci Med, Prof.
8 bldg. 2, Trubetskaya Str., Moscow 119991
Scopus Author ID 6505479504; WoS ResearcherID Q-1385-2015
J. Kh. Khizroeva
Russian Federation
Jamilya Kh. Khizroeva - MD, Dr Sci Med, Prof.
8 bldg. 2, Trubetskaya Str., Moscow 119991
Scopus Author ID 57194547147; WoS ResearcherID F-8384-2017
L. A. Ashrafyan
Russian Federation
Lev A. Ashrafyan - MD, Dr Sci Med, Prof., Academician of RAS.
4 Academika Oparina Str., Moscow 117997
Scopus Author ID 57194173388
V. N. Serov
Russian Federation
Vladimir N. Serov - MD, Dr Sci Med, Prof., Academician of RAS.
4 Academika Oparina Str., Moscow 117997
A. Е. Voynovskiy
Russian Federation
Alexander Е. Voynovskiy - MD, Dr Sci Med, Prof.
2/44 Salyama Adilya Str., Moscow 123423
WoS ResearcherlD S-6385-2016
J. Yu. Ungiadze
Georgia
Jumber Yu. Ungiadze - MD, Dr Sci Med, Prof.
35 Ninoshvili Str., Batumi, Autonomous Republic of Adjara 6010
A. V. Lazarchuk
Russian Federation
Arina V. Lazarchuk.
8 bldg. 2, Trubetskaya Str., Moscow 119991
M. V. Tretyakova
Russian Federation
Maria V. Tretyakova - MD, PhD.
8 bldg. 2, Trubetskaya Str., Moscow 119991
N. A. Makatsariya
Russian Federation
Nataliya A. Makatsariya - MD, PhD.
8 bldg. 2, Trubetskaya Str., Moscow 119991
WoS ResearcherID F-8406-2017
P. V. Salnikova
Russian Federation
Polina V. Salnikova.
8 bldg. 2, Trubetskaya Str., Moscow 119991
N. R. Gashimova
Russian Federation
Nilufar R. Gashimova - MD.
8 bldg. 2, Trubetskaya Str., Moscow 119991
K. N. Grigoreva
Russian Federation
Kristina N. Grigoreva - MD.
8 bldg. 2, Trubetskaya Str., Moscow 119991
K. L. Zakashansky
United States
Konstantin L. Zakashansky - MD.
1176 Fifth Avenue, Box 1173, New York 10029-6574
I. Elalamy
Russian Federation
Ismail Elalamy - MD, Dr Sci Med, Prof.
8 bldg. 2, Trubetskaya Str., Moscow 119991; 12 Rue de Г Ecole de Medecine, Paris 75006, France; 4 Rue de la Chine, Paris 75020, France
Scopus Author ID 7003652413; WoS ResearcherID AAC-9695-2019
J.-C. Gris
Russian Federation
Jean-Christophe Gris - MD, Dr Sci Med, Prof.
8 bldg. 2, Trubetskaya Str., Moscow 119991; 163 Rue Auguste Broussonnet, Montpellier 34090, France
Scopus Author ID 7005114260; WoS ResearcherID AAA-2923-2019
Review
For citations:
Makatsariya A.D., Slukhanchuk E.V., Bitsadze V.O., Solopova A.G., Khizroeva J.Kh., Ashrafyan L.A., Serov V.N., Voynovskiy A.Е., Ungiadze J.Yu., Lazarchuk A.V., Tretyakova M.V., Makatsariya N.A., Salnikova P.V., Gashimova N.R., Grigoreva K.N., Zakashansky K.L., Elalamy I., Gris J. The concept of thromboinflammation underlying thrombotic complications, tumor progression and metastasis in gynecological cancer patients. Obstetrics, Gynecology and Reproduction. 2024;18(4):450-463. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.542

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































