Scroll to:
NOD-like receptors in pathogenesis of missed and spontaneous abortions
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.435
Abstract
Nucleotide-binding oligomerization domain-like receptors (NOD-like receptors) are cytosolic signaling receptors of innate immune cells recognizing ligands derived from bacteria, viruses, fungi and protozoa. They can initiate apoptosis and pro-inflammatory cytokines production. Meanwhile, the role of decidual NOD-like receptors in pathogenesis of early miscarriages remains unknown.
Aim: to study NOD-like receptor (NOD1, NOD2, NLRP1, NLRP3, NLRC4) messenger ribonucleic acid (mRNA) expression in decidual tissue from patients with missedand spontaneous abortions compared to progressive pregnancy.
Materials and Methods. NOD1, NOD2, NLRP1, NLRP3, NLRC4 and pathway protein receptorinteracting-serine/threonine-protein kinase 2 (RIP-2) mRNA expression in decidua from 34 patients with missed abortions (group I), 34 patients with spontaneous abortions (group II) and 57 women with progressive pregnancy admitted for artificial abortion (group III, control group) were analyzed by reverse transcription quantitative polymerase chain reaction (PCR) at gestational age of 6–10 weeks. Exclusion criteria were as follows: endocrine disorders, severe extragenital diseases, antiphospholipid syndrome, inherited thrombophilia, uterine malformations and fetal chromosomal abnormalities. Samples were collected by uterine abrasion.
Results. It was found that mRNA expression of NOD2 was significantly higher in decidua from patients with missed and spontaneous abortions, whereas for RIP-2 (related to relevant signaling pathway) – in women with missed abortions. A moderate positive correlation between gestational age and mRNA expression for NOD2 (R = 0.48; p = 0.01) and RIP-2 (R = 0.41; p = 0.007) was observed in subjects with progressive pregnancy. In contrast, women with missed abortions showed a moderate negative correlation between body weight and mRNA expression for NOD2 (R = –0.46; p = 0.03) and RIP-2 (R = –0.51; p = 0.02) whereas spontaneous abortions was associated with moderate negative correlation between RIP-2 mRNA expression and body weight (R= –0.47; p=0.04) as well as body mass index (R= –0.48; p = 0.04) along with moderate positive correlation with age of menarche (R = 0.46; p = 0.04). However, compared with progressive pregnancy no significant differences were found in expression level form NOD1, NLRP1, NLRP3 and NLRC4 mRNA in decidua from patients with missed and spontaneous abortions.
Conclusion. Elevated NOD2 mRNA expression was observed in decidua from patients with missed and spontaneous abortions compared to progressive pregnancy paralleled with upregulated RIP-2 mRNA expression in missed abortions. Finally, it was found that NOD1, NLRP1, NLRP3 and NLRC4 were not involved in pathogenesis of miscarriages.
Keywords
For citations:
Lebedeva O.P., Ivannikova V.M., Zhukova I.O., Kozarenko O.N., Altukhova O.B., Pakhomov S.P., Churnosov M.I. NOD-like receptors in pathogenesis of missed and spontaneous abortions. Obstetrics, Gynecology and Reproduction. 2023;17(5):554-564. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.435
Introduction / Введение
The unique feature of female reproductive tract is its ability to provide defense against pathogens and immune tolerance to semiallogeneic fetus. It is known that excessive immune response to exogenous and endogenous stimuli can lead to miscarriage [1].
Initial pathogen recognition is provided by innate immune signalling receptors, among which the role of Toll-like receptors in the pathogenesis of early miscarriages has been largely studied [2–6]. NOD-like receptors are positioned in the cellular cytoplasm and can recognize viruses, bacteria, protozoa and fungi in the female genital tract as well as participate in inducing apoptosis and inflammation (Fig. 1) [7]. At the same time, no data on the role of NOD-like receptors in decidual tissue in pathogenesis of early miscarriages are currently available.
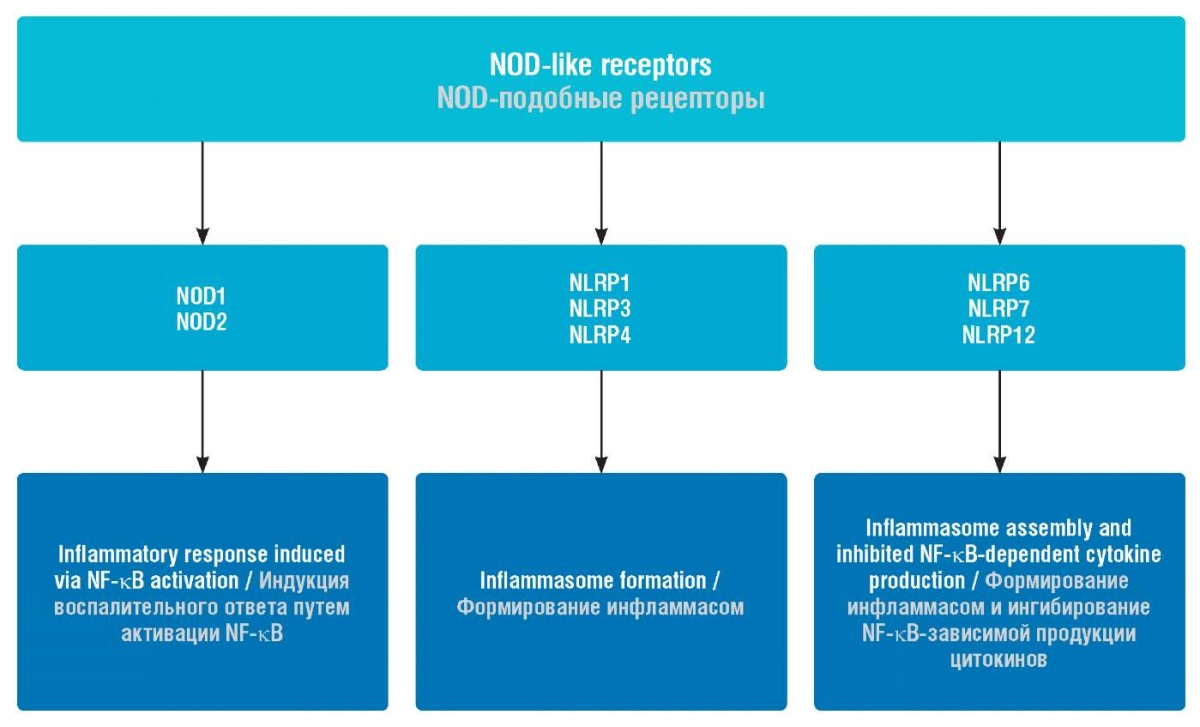
Figure 1. Functions of NOD-like receptors.
Note: NOD – nucleotide-binding oligomerization domain;
NLRP – nucleotide-binding oligomerization domain,
leucine rich repeat and pyrin domain containing;
NLRC – NLR family, CARD domain containing;
NF-κB –nuclear factor kappa-B.
Рисунок 1. Функции NOD-подобных рецепторов.
Примечание: NOD – нуклеотид-связывающий домен олигомеризации;
NLRP – нуклеотид-связывающий домен олигомеризации,
содержащий обогащенные лейцином повторы и пириновый домен;
NLRC – рецептор, относящийся к семейству NOD-подобных рецепторов,
содержащий домен CARD;
NF-κB – нуклеарный фактор каппа-B.
Aim: to study NOD-like receptor (NOD1, NOD2, NLRP1, NLRP3, NLRC4) messenger ribonucleic acid (mRNA) expression in decidual tissue from patients with missed and spontaneous abortions compared to progressive pregnancy.
Materials and Methods / Материалы и методы
Study design / Дизайн исследования
Observational prospective cross-sectional comparative cohort study was performed in the Perinatal Center of Belgorod Regional Clinical Hospital of St. Joasaph. Quantitative reverse-transcriptase polymerase chain reaction (PCR) was carried out in the Laboratory of Human Molecular Genetics, Belgorod National Research University. Samples were collected from January 2017 till January 2018. Currently, artificial abortions in early pregnancy are provided only by administered medications (mifepristone + misoprostol), therefore not allowing to collect endometrial samples by curettage of the uterine cavity at this gestational age. Hence, there were used complementary DNA (cDNA) from previously collected samples. After extraction and reverse transcription, cDNA was stored at –80 °C (to store cDNA for unlimited time) until further investigated.
Comparison groups / Группы сравнения
Total 125 women (aged 20–35 years old) were examined at a single time point (6–10 weeks of gestation). Women were divided into three groups: group I – 34 patients with missed abortions, 27.88 ± 1.07 years old, 7.50 ± 0.39 weeks of gestation; group II – 34 patients with spontaneous abortions, 27.05 ± 0.86 years old, 7.44 ± 0.37 weeks of gestation; group III (control group) – 57 women with ongoing (progressive) pregnancy, admitted to the Perinatal Center for artificial abortion, 26.46 ± 0.52 years old, 8.23 ± 0.18 weeks of gestation. Baseline clinical characteristics were recorded for all subject groups.
Inclusion and exclusion criteria / Критерии включения и исключения
Inclusion criteria for group I: gestational age – from 6 to 10 weeks; clinical picture of missed abortion (crown-rump length exceeds 7 mm, in case no embryo heart-beat, or mean gestational sac diameter is more than 25 mm in absence of embryo, or embryo with heartbeat is absent for more than 14 days after ultrasound detection of gestational sac without yolk sac, or embryo with heartbeat is absent 10 days after ultrasound examination detecting gestational sac with yolk sac).
Inclusion criteria for group II: gestational age – from 6 to 10 weeks; clinical picture of spontaneous abortion (presence of the gestational sac completely detached from uterine wall, after previously detected progressive pregnancy with embryo heartbeat, regular contractions of shortened cervix myometrium, dilation of internal and internal cervical os, expulsion of gestational sac from uterine cavity).
Inclusion criteria for group III: gestational age – from 6 to 10 weeks; ongoing uterine pregnancy, detection of embryo heartbeat; a woman admitted for artificial abortion by uterine abrasion, written and signed consent for uterine abrasion was obtained.
Exclusion criteria: fetal chromosomal abnormalities; uterine malformations; severe extragenital diseases; endocrine disorders; inherited thrombophilia; antiphospholipid syndrome; refuse to sign patient’s consent.
The diagnosis was made based on clinical data and ultrasound examination data to be further confirmed by histopathological examination.
Study methods / Методы исследования
Messenger RNA expression for NOD1, NOD2, NLRP1, NLRP3, NLRC4 as well as related signalling pathway protein RIP-2 (receptor-interacting-serine/threonine-protein kinase 2) in decidual samples was assessed by PCR according to MIQE (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) guidelines [8].
Epithelial tissue samples were obtained by uterine abrasion to be stored in RNAlater solution (Thermo Fisher Scientific, Madison, WI, USA).
Trisol (Invitrogen, Waltham, MA, USA) was used for RNA isolation followed by running ethidium bromide-containing agarose gel electrophoresis for quality control. Next, RNA samples were treated with by DNase I (RNase free) kit (Thermo Fisher Scientific, Madison, WI, USA) to avoid genomic DNA contamination. Reverse transcriptase Mint kit was used to run reverse transcription added with 20 μM of oligoDT (Evrogen, Moscow, Russia) and 500 ng of RNA using PCR Thermal Cycler “Tercyc” (DNA Technology, Moscow, Russia). cDNA quality was analysed in ethidium bromide-containing agarose gel electrophoresis.
PCR primers were designed using NCBI (National Center for Biotechnology Information) database (Bethesda, MD, USA) (Table 1).
Table 1. Primers for quantitative polymerase chain reaction.
Таблица 1. Праймеры для количественной полимеразной цепной реакции.
|
Gene Ген |
Accession number Код доступа |
Product length, basic pairs Длина продукта, пары оснований |
Forward primer 5'-3' Прямой праймер 5'-3' |
Reverse primer 5'-3' Обратный праймер5'-3' |
Annealing temperature, ºC Температура отжига, ºC |
|
NOD1 |
NM_006092.4 |
821 |
CCTGGTGGCCAAGTGATTGT |
ACCGAAGGAAATTGCCATCAAAG |
55 |
|
NOD2 |
NM_022162.2 |
240 |
CTAATGGGCTTTGATGGGGGAA |
AGGTGGAAGCCCTCGTAGT |
55 |
|
NLRP1 |
NM_033004.4 |
942 |
TACCGGTGGAACTCTTGTGC |
GGGCTGGAGGGATCAGAGTA |
55 |
|
NLRP3 |
NM_004895.4 |
570 |
CTGAGCTGACCGTCGTCTTT |
AACCAGCTACAAAAAGCATGGAT |
64 |
|
NLRC4 |
NM_021209.4 |
949 |
CCTGCTGACTGAGAGAACACA |
GGCAGTTCTGGGGCTTGAAT |
55 |
|
RIP-2 |
NM_003821.5 |
374 |
TGCTCGACAGTGAAAGAAAGGA |
TCGTGACTGTGAGAGGGACA |
55 |
|
β-actin |
NM_001101.5 |
994 |
CAGGCACCAGGGCGTGATGG |
GATGGAGGGGCCGGACTCGT |
64 |
|
PPIA |
NM_021130.5 |
327 |
CCGCCGAGGAAAACCGTGTACT |
TGGACAAGATGCCAGGACCCGT |
64 |
Note: NOD – nucleotide-binding oligomerization domain;
NLRP – nucleotide-binding oligomerization domain,
leucine rich repeat and pyrin domain containing;
NLRC4 – NLR family, CARD domain containing 4;
RIP-2 – receptor-interacting-serine/threonine-protein kinase 2 (RIPK2 or RIP2);
PPIA – peptidylprolylisomerase A.
Примечание: NOD – нуклеотид-связывающий домен олигомеризации;
NLRP – нуклеотид-связывающий домен олигомеризации,
содержащий обогащенные лейцином повторы и пириновый домен;
NLRC4 – рецептор, относящийся к семейству NOD-подобных рецепторов,
содержащий домен CARD4;
RIP-2 – рецептор, взаимодействующий с серин/треонинкиназой 2;
PPIA – пептидилпролилизомераза A.
qPCR-mix HS SYBR kit (Evrogen, Moscow, Russia) was used for amplification reaction using amplifier CFX96 (Bio-Rad, Hercules, CA, USA) as follows: 14 μL of sterile water, 2 μL of forward and reverse primers each, 5 μL of qPCRmix HS SYBR, and 2 μL of cDNA sample (in triplicates). The amplification cycle included: 5 minutes at 95 °C, followed by 45 three-step cycles (15 seconds at 95 °С, 30 seconds at annealing temperature according, see Table 1, and 30 seconds at 68 °C). Two housekeeping genes – peptidylprolylisomerase А (PPIA) and β-actin were used as reference genes.
The results were calculated and presented in relative units as 2–ΔΔCq using the formula:
R = 2–(Cq target- (Cq ref1 + Cq ref2)/2),
where R – normalized mRNA expression of study gene; Cq ref1 and Cq ref2 – Cq of housekeeping genes; Cq target – Cq of study gene [9].
Ethical aspects / Этические аспекты
The study was designed in accordance with the ethical standards of the Declaration of Helsinki of the World Medical Association (1964) and its subsequent amendments and comparable ethical standards. All patients signed written consent for collection of samples and clinical data, including permission for publication without personal data. The study was approved by Ethics Committee of Belgorod Regional Clinical Hospital of St. Joasaph, Belgorod, Russia (Protocol No. 15 dated of December 21, 2016).
Statistical analysis / Статистический анализ
Statistical analysis was performed by using Statistica 13.2 (Statsoft Inc., Tulsa, OK, USA) and GraphPad Prism 8.0 (Dotmatics, Boston, MA, USA) software. Normality of distribution was estimated by Kolmogorov–Smirnov test. If normal distribution was found, data were presented as M ± SEM and inter-group differences were estimated by t-test for independent samples. If non-normal distribution was detected, result values were represented as median with 95 % confidence interval (CI) and inter-group differences were estimated by Mann–Whitney U-test. Correlation analysis was performed using Spearman's rank criterion. Differences were considered significant at p-value ≤ 0.05.
Results / Результаты
Clinical and anamnestic characteristics of the groups examined / Клинико-анамнестическая характеристика обследованных групп
All studied groups were compatible by age, gestational age, and morphometric parameters – weight, height, and body mass index (BMI). No significant differences in anamnesis data were found between all studied groups, except pregnancy outcomes (Table 2).
Table 2. Clinical and anamnestic characteristics
of the women examined (M ± SEM).
Таблица 2. Клинико-анамнестическая характеристика
обследованных женщин (M ± SEM).
|
Parameter Показатель |
Group I Группа I n = 34 |
Group II Группа II n = 34 |
Group III Группа III n = 57 |
р1–3 |
р2–3 |
р1–2 |
|
Demographic characteristics / Демографические характеристики |
||||||
|
Age, years Возраст, лет |
27.88 ± 1.07 |
27.05 ± 0.86 |
26.46 ± 0.52 |
0.19 |
0.56 |
0.55 |
|
Gestational age, weeks Срок беременности, недель |
7.50 ± 0.39 |
7.50 ± 0.39 |
8.23 ± 0.18 |
0.06 |
0.06 |
0.99 |
|
Height, cm Рост, см |
162.96 ± 1.27 |
165.95 ± 1.31 |
163.24 ± 1.42 |
0.90 |
0.26 |
0.11 |
|
Weight, kg Масса тела, кг |
61.16 ± 2.00 |
65.36 ± 3.40 |
63.69 ± 1.61 |
0.36 |
0.62 |
0.29 |
|
Body mass index, kg/m² Индекс массы тела, кг/м² |
23.01 ± 0.75 |
23.64 ± 1.10 |
24.21 ± 0.80 |
0.38 |
0.70 |
0.66 |
|
Anamnesis data / Данные анамнеза |
||||||
|
Age of menarche, years Возраст менархе, лет |
13.00 ± 0.32 |
12.77 ± 0.30 |
12.68 ± 0.14 |
0.29 |
0.76 |
0.61 |
|
Duration of menstrual cycle, days Длительность менструального цикла, дней |
28.00 ± 0.40 |
27.71 ± 0.39 |
28.04 ± 0.19 |
0.92 |
0.71 |
0.61 |
|
Duration of menstruation, days Длительность менструации, дней |
4.74 ± 0.13 |
4.89 ± 0.13 |
4.89 ± 0.09 |
0.33 |
0.41 |
0.25 |
|
Total number of pregnancies, including: Общее количество беременностей, включая: childbirth / роды medical abortions / медицинские аборты spontaneous abortions / самопроизвольные выкидыши missed abortions / неразвивающаяся беременность |
2.83 ± 0.41 0.96 ± 0.26 1.08 ± 0.19 0.16 ± 0.09 0.63 ± 0.13 |
2.77 ± 0.35 0.77 ± 0.17 1.00 ± 0.20 0.50 ± 0.13 0.50 ± 0.13 |
2.84 ± 0.22 1.10 ± 0.10 1.71 ± 0.16 0.03 ± 0.02 – |
0.92 0.58 0.02 0.11 0.001 |
0.87 0.12 0.01 0.001 0.001 |
0.96 0.56 0.77 0.03 0.50 |
Note: р1–3 – significant differences between group I and the control group;
р2–3 – significant difference between group II and the control group;
р1–2 – significant difference between group I and group II;
significant differences are highlighted in bold.
Примечание: р1–3 – статистическая значимость различий
между группой I и контрольной группой;
р2–3 – статистическая значимость различий
между группой II и контрольной группой;
р1–2 – статистическая значимость различий между группами I и II;
выделены значимые различия.
NOD-like receptor mRNA expression in decidual tissue / Экспрессия мРНК NOD-подобных рецепторов в децидуальной ткани
NOD2 mRNA expression in decidual tissue from patients with spontaneous abortions and missed abortions was significantly higher compared to subjects with ongoing pregnancy (Fig. 2). In women with missed abortions vs. ongoing pregnancy, it was accompanied by elevated RIP-2 mRNA expression.
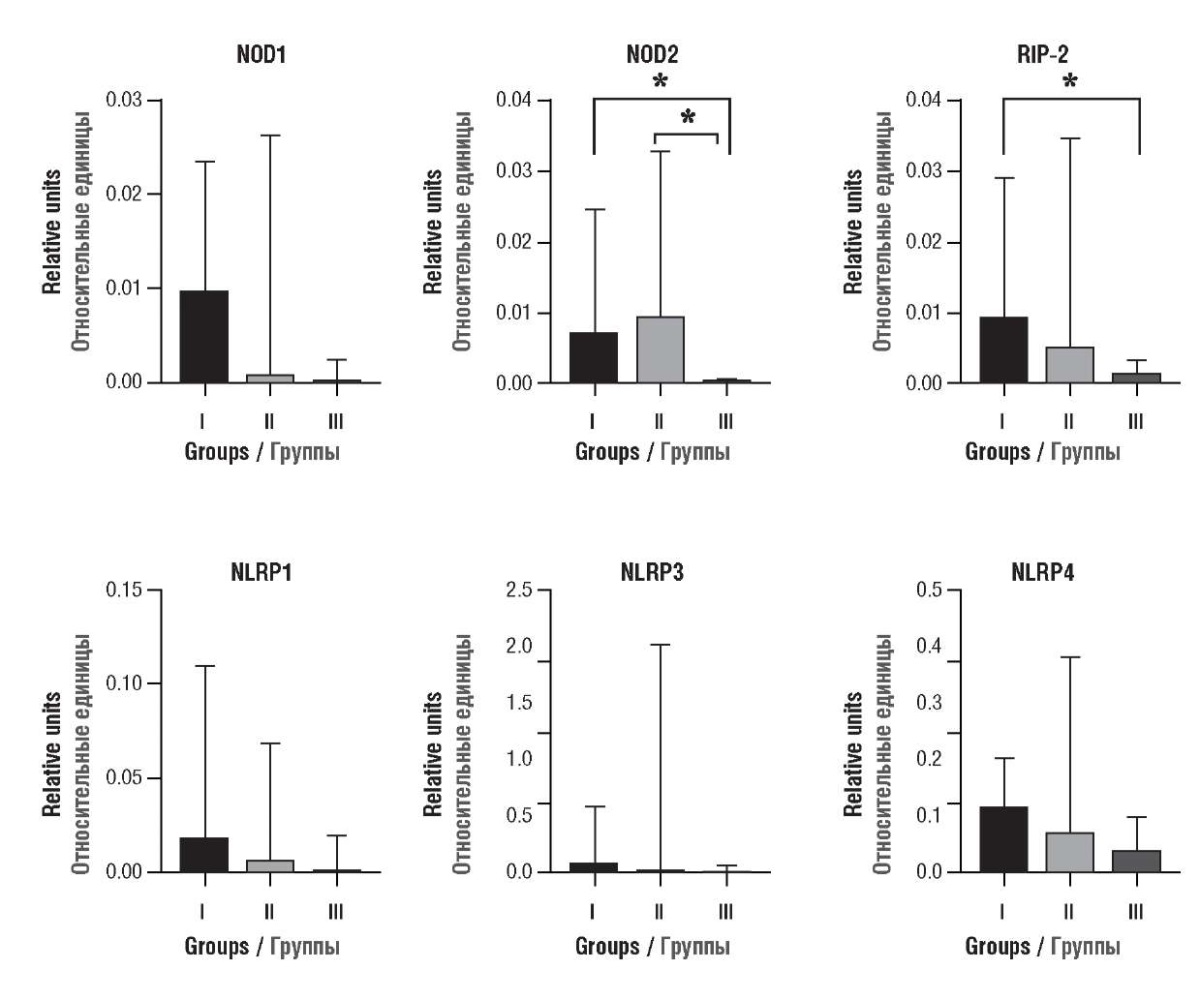
Figure 2. Comparison of NLRs mRNA expression in decidual tissue
from patients with missed abortions (group I, n = 34),
spontaneous abortions (group II, n = 34),
and women with ongoing pregnancy admitted for artificial abortions (group III, n = 57).
Data are presented as median with 95 % confidence interval.
Note: *p < 0.05 – significant inter-group differences;
NOD1 – nucleotide-binding oligomerization domain 1;
NOD2 – nucleotide-binding oligomerization domain 2;
RIP-2 – receptor-interacting-serine/threonine-protein kinase 2;
NLRP 1 – nucleotide-binding oligomerization domain,
leucine rich repeat and pyrin domain containing 1;
NLRP 3 – nucleotide-binding oligomerization domain,
leucine rich repeat and pyrin domain containing 3;
NLRC4 – NLR family, CARD domain containing 4.
Рисунок 2. Сравнение экспрессии мРНК в децидуальной ткани
у пациенток с неразвивающейся беременностью (группа I, n = 34),
самопроизвольными выкидышами, (группа II, n = 34)
и женщинами с прогрессирующей беременностью,
поступившими для медицинского аборта (группа III, n = 57).
Данные представлены как медиана и 95 % доверительный интервал.
Примечание: *p< 0.05 – значимые различия между группами;
NOD1 – нуклеотид-связывающий домен олигомеризации 1;
NOD2 – нуклеотид-связывающий домен олигомеризации 2;
RIP-2 – рецептор, взаимодействующий с серин/треонинкиназой 2;
NLRP1 – нуклеотид-связывающий домен олигомеризации,
содержащий обогащенные лейцином повторы и пириновый домен;
NLRP3 – нуклеотид-связывающий домен олигомеризации,
содержащий обогащенные лейцином повторы и пириновый домен 3;
NLRC4 – рецептор, относящийся к семейству NOD-подобных рецепторов,
содержащий домен CARD4.
mRNA expression of NOD1 and NOD-like receptors involved in inflammasome assembly revealed no significant differences between all studied groups.
We estimated a correlation between decidual mRNA expression level for NOD2 and RIP-2 and clinical data (Table 2).
It was found, that patients with progressive pregnancy (control group) showed only moderate positive correlation between gestational age and expression level for NOD2 (R = 0.48; p = 0.01) and RIP-2 (R = 0.41; p = 0.007) mRNA.
Patients with missed abortions displayed a moderate negative correlation between body weight and mRNA expression for NOD2 (R = –0.46; p = 0.03) and RIP-2 (R = –0.51; p = 0.02).
Patients with spontaneous abortion showed no correlation between expression level for NOD2 mRNA and presented clinical characteristics. However, expression of RIP-2 mRNA had a moderate negative correlation with the body weight (R = –0.47; p = 0.04), but demonstrated a moderate positive correlation with age of menarche (R = 0.46; p = 0.04).
Discussion / Обсуждение
According to the recent microbiome studies, bacterial pathogens represent the major causative cues in early-stage miscarriages [10][11]. Therefore, a decidual inflammation induced via NOD-like receptors is of the great interest.
NOD2 is a cytosolic receptor becoming activated after binding to muramyl dipeptide, the major component of peptidoglycan (the part of Gram-negative and Gram-positive bacteria) [12]. The main pathway protein for NOD2 intracellular signal transduction is presented by RIP-2 capable to activate NF-κB and mitogen-activated protein kinase (MAPK) and resulting in production of pro-inflammatory cytokines, chemokines and antimicrobial peptides [13].
NOD2 also recognizes viral RNA leading to production of interferon-β and activation of antiviral adapter protein mitochondrial antiviral-signaling protein (MAVS). NOD2 also triggers 2'-5'-oligoadenylate synthetase 2 (OAS2), activating RNAse L leading to degradation of viral RNA [14]. NOD2 ensures autophagy of infected cells, which is important for elimination of viruses, bacteria, and protozoa [15] as well as for antigen presentation [16].
The expression of NOD2 mRNA and protein was detected in the decidualized stroma and glandular epithelium in early ongoing pregnancy [17][18]. It was found that in vitro stimulation of decidualized endometrial stromal cells by muramyl dipeptide upregulates expression of NOD2 mRNA and protein as well as further promotes secretion of monocyte chemoattractant protein 1 (MCP-1) and interleukin-1β (IL-1β). IL-1β, in turn, can stimulate NOD2, which might potentiate inflammation via a positive feedback loop. Stimulation by muramyl dipeptide also results in dose-dependent induction of apoptosis in decidualized stroma cells [18].
Currently, no data regarding NOD2 expression in decidual tissue from patients with spontaneous abortions and missed abortions are available. Patients with vs. without history of unexplained recurrent miscarriages had downregulated NOD2 expression at the level of mRNA and protein [19]. However, the exclusion criteria in the study included concomitant infection. In addition, patients in both main and control group were admitted for medical abortion inferring that during sample collection all of them had a progressive (ongoing) pregnancy.
In another study, NOD1 and NOD2 mRNA and protein expression was elevated in trophoblast from patients with recurrent miscarriages compared to normal ongoing pregnancy [20]. Meanwhile, no data on decidual NOD1 expression in patients with miscarriage were published. It is known that activation of NOD1 occurs upon binding to diaminopimelic acid (iE-DAP), a part of the peptidoglycan found in some Gram-positive and all Gram-negative bacteria [12].
It can be suggested that upregulated decidual mRNA expression for NOD2, but not NOD1, in patients with spontaneous abortions and missed abortions might be due to Gram-positive, rather than Gram-negative bacteria present in the decidual tissue.
In addition, no data regarding a role for NLRP1 and NLRC4 in pathogenesis of early miscarriages are found. We described no significant differences in decidual expression level for NLRP1 and NLRC4 mRNA in patients with spontaneous abortions and missed abortions.
Ligands for NLRP1 are presented by muramyl dipeptide and anthrax lethal toxin. Expression of NLRP1 mRNA is regulated by protein SREBF1 regardless of pro-inflammatory cytokine levels [21]. It is known, that NLRP1 binding to apoptosis-regulating proteins B-cell lymphoma 2 apoptosis regulator (BCL-2) and B-cell lymphoma-extra large apoptosis regulator (BCL-XL) attenuates inflammasome formation [22][23]. Earlier, normal or elevated decidual expression of BCL-2 mRNA was identified in patients with spontaneous abortions or missed abortions, respectively [24]. Apparently, this may prevent the formation of NLRP1 inflammasome and induction of apoptosis in the decidual tissue.
The main ligands for human NLRC4 are presented by bacterial flagellin and structural proteins of bacterial type III secretion system [25][26].
In our study, no significant differences were found in NLRP3 mRNA expression in both patient groups compared to ongoing pregnancy group. NLRP3 detects the whole spectrum of bacterial, fungal, protozoan, and viral ligands, as well as a number of host damage-associated molecular patterns [26]. In nonpregnant patients with vs. without history of recurrent miscarriages of unknown origin, during the secretory phase of menstrual cycle endometrium was discovered to have upregulated expression of NLRP3 protein [1], but not mRNA [27]. Higher NLRP3 mRNA expression was found in peripheral blood mononuclear cells from patients with history of recurrent miscarriage compared to healthy women [28]. Further research is required to assess a role of NLRP3 in the pathogenesis of miscarriages.
Patients with progressive pregnancy we found to show a significant positive correlation between decidual expression level of NOD2 and RIP-2 mRNA and gestational age suggesting that NOD2 level normally escalates in parallel with increasing gestational age.
Patients with missed abortions had significant negative correlation between NOD2 and RIP-2 mRNA expression and body weight, whereas patients with spontaneous abortions – between RIP-2 mRNA expression and body weight as well as body mass index. No data regarding features of NOD2 and RIP-2 expression in human decidua with regard to body weight were found. However, NOD2-deficient mice were shown to display insulin resistance and higher level of adipose tissue [29].
Patients with spontaneous miscarriages had also a positive correlation observed between decidual RIP-2 mRNA expression and age of menarche onset. It is known that higher BMI is associated with earlier menarche [30]. Therefore, patients with low body weight usually have menarche onset at older age. Thus, it is consistent to find a negative correlation between RIP-2 mRNA expression and body weight as well as BMI, and its positive correlation with age of menarche onset.
Study limitations / Ограничения исследования
Limitations of the study were accounted for by a relatively small quantity of samples and a single-center study design conducted only in a single region of Russian Federation. Multicenter studies, especially those involving multiple countries, can provide more comprehensive insights into a role of NOD-like receptors in pathogenesis of miscarriages.
Conclusion / Заключение
In summary, upregulated NOD2 mRNA expression is observed in decidua from patients with spontaneous abortions and missed abortions compared to progressive pregnancy resulting in increased RIP-2 mRNA expression level in missed abortions. NOD1, NLRP1, NLRP3 and NLRC4 were found not to be involved in miscarriage pathogenesis.
References
1. D’Ippolito S., Tersigni C., Marana R. et al. Inflammosome in the human endometrium: further step in the evaluation of the “maternal side”. Fertil Steril. 2016;105(1):111–118.e1–4. https://doi.org/10.1016/j.fertnstert.2015.09.027.
2. Лебедева О.П., Кирко Р. Экспрессия толл-подобных рецепторов в женском репродуктивном тракте и ее гормональная регуляция (обзор). Научные результаты биомедицинских исследований. 2018;4(3):3–17.
3. Lebedeva O.P., Pakhomov S.P., Ivashova O.N. et al. Expression of TLR 1-10 and caspase-3 alfa in human endometrium at women with early miscarriages. Giornale Italiano di Ostetricia e Ginecologia. 2013;35(1):270–1.
4. Kolben T.M., Rogatsch E., Hester A. et al. Involvement of ILR4α and TLR4 in miscarriages. J Reprod Immunol. 2019;131:36–43. https://doi.org/10.1016/j.jri.2018.12.001.
5. Лебедева О.П., Жукова И.О., Ивашова О.Н. и др. Роль рецепторов RIG-I, AIM2 и IFI16, распознающих вирусную ДНК и РНК, в патогенезе самопроизвольных выкидышей и неразвивающейся беременности ранних сроков. Акушерство и гинекология. 2018;(7):57–61. https://doi.org/10.18565/aig.2018.7.57-61.
6. Лебедева О.П., Ивашова О.Н., Пахомов С.П. и др. Невынашивание беременности как проблема иммунного конфликта. Проблемы репродукции. 2014;(6):88–91.
7. Лебедева О.П. Роль рецепторов NOD1 и NOD2 в распознавании патогенов в женском репродуктивном тракте. Акушерство и гинекология. 2019;(5):25–9. https://doi.org/10.18565/aig.2019.5.25-29.
8. Bustin S.A., Benes V., Garson J.A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–22. https://doi.org/10.1373/clinchem.2008.112797.
9. Pffafl M.W. Quantification strategies in real-time PCR. In: AZ of quantitative PCR. Ed. S.A. Bustin. Intenational University Line, 2004. Chapter 3. 87–112. Available at: https://www.gene-quantification.de/ chapter-3-pfaffl.pdf. [Accessed: 15.07.2023].
10. Lebedeva O.P., Popov V.N., Syromyatnikov M.Y. et al. Female reproductive tract microbiome and early miscarriages. APMIS. 2023;131(2):61–76. https://doi.org/10.1111/apm.13288.
11. Лебедева О.П., Грязнова М.В., Козаренко О.Н. и др. Микробиом влагалища при нарушениях менструального цикла (обзор). Научные результаты биомедицинских исследований. 2021;7(4):433–50. https://doi.org/10.18413/2658-6533-2021-7-4-0-9.
12. Philpott D.J., Girardin S.E. Nod-like receptors: sentinels at host membranes. Curr Opin Immunol. 2010;22(4):428–34. https://doi.org/10.1016/j.coi.2010.04.010.
13. Saxena M., Yeretssian G. NOD-like receptors: master regulators of inflammation and cancer. Front Immunol. 2014;5:327. https://doi.org/10.3389/fimmu.2014.00327.
14. Lupfer C., Kanneganti T.D. Unsolved mysteries in NLR biology. Front Immunol. 2013;4:285. https://doi.org/10.3389/fimmu.2013.00285.
15. Mukherjee T., Hovingh E.S., Foerster E.G. et al. NOD1 and NOD2 in inflammation, immunity and disease. Arch Biochem Biophys. 2019;670:69–81. https://doi.org/10.1016/j.abb.2018.12.022.
16. Trindade B.C., Chen G.Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol Rev. 2020;297(1):139–61. https://doi.org/10.1111/ imr.12902.
17. King A.E., Horne A.W., Hombach-Klonisch S. et al. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. 2009;15(5):311–9. https://doi.org/10.1093/molehr/gap020.
18. Zhang Y. Chen H., Sun C. et al. Expression and functional characterization of NOD2 in decidual stromal cells Isolated during the first trimester of pregnancy. PLoS One. 2014;9(6):e99612. https://doi.org/10.1371/journal.pone.0099612.
19. Zhang Y., Yang C., Fu S. et al. Different expression of NOD2 in decidual stromal cells between normal and unexplained recurrent spontaneous abortion women during first trimester gestation. Int J Clin Exp Pathol. 2014;7(12):8784–90.
20. Wang Z., Liu M., Nie X. et al. NOD1 and NOD2 control the invasiveness of trophoblast cells via the MAPK/p38 signaling pathway in human firsttrimester pregnancy. Placenta. 2015;36(6):652–60. https://doi.org/10.1016/j.placenta.2015.03.004.
21. Im S.S., Yousef L., Blaschitz C. et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13(5):540–49. https://doi.org/10.1016/j.cmet.2011.04.001.
22. Bruey J.M., Bruey-Sedano N., Luciano F. et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129(1):45–56. https://doi.org/10.1016/j.cell.2007.01.045.
23. Faustin B., Chen Y., Zhai D. et al. Mechanism of Bcl-2 and Bcl-XL inhibition of NLRP1 inflammasome: Loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci U S A. 2009;106(10):3935–40. https://doi.org/10.1073/pnas.0809414106.
24. Lebedeva O.P., Zhukova I.O., Ivashova O.N. et al. Proteins P53 and BCL-2 in pathogenesis of missed and spontaneous abortions. Drug Invention Today. 2017;9(3):65–8.
25. Fusco W.G., Duncan J.A. Novel aspects of the assembly and activation of inflammasomes with focus on the NLRC4 inflammasome. Int Immunol. 2018;30(5):183–93. https://doi.org/10.1093/intimm/dxy009.
26. de Zoete M.R., Palm N.W., Zhu S. et al. Inflammasomes. Cold Spring Harb Perspect Biol. 2014;6(12):a016287. https://doi.org/10.1101/cshperspect.a016287.
27. Grasso E., Gori S., Soczewski E. et al. Impact of the Reticular Stress and Unfolded Protein Response on the inflammatory response in endometrial stromal cells. Sci Rep. 2018;8(1):12274. https://doi.org/10.1038/s41598- 018-29779-8.
28. Lu M., Ma F., Xiao J. et al. NLRP3 inflammasome as the potential target mechanism and therapy in recurrent spontaneous abortions. Mol Med Reps. 2019;19(3):1935–41. https://doi.org/10.3892/mmr.2019.9829.
29. Denou E., Lolmède K., Garidou L. et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med. 2015;7(3):259–74. https://doi.org/10.15252/emmm.201404169.
30. Anderson B.L., Simhan H.N., Simons K.M., Wiesenfeld H.C. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. Am J Obstet Gynecol. 2007;196(6):524.e1–5. https://doi.org/10.1016/j.ajog.2007.01.006.
About the Authors
O. P. LebedevaRussian Federation
Olga P. Lebedeva – MD, Dr Med Sci, Associate Professor, Professor, Department of Obstetrics and Gynecology; Leading Researcher, Laboratory of Metagenomics and Food Biotechnology
Scopus Author ID: 55655876400
Researcher ID: E-5969-2015
85 Pobedy Str., Belgorod 308015
19 Prospect Revolutsii, Voronezh 394036
V. M. Ivannikova
Russian Federation
Victoria M. Ivannikova – MD, Postgraduate Student, Department of Obstetrics and Gynecology; Ultrasound Doctor, Prenatal Diagnostics Room
85 Pobedy Str., Belgorod 308015
2 Hospital Square, Moscow 111020
I. O. Zhukova
Russian Federation
Irina O. Zhukova – MD, Postgraduate Student, Department of Obstetrics and Gynecology; Obstetrician-Gynecologist
85 Pobedy Str., Belgorod 308015
81 Proletarskaya Str., village Rakitnoe, Belgorod region 309310
O. N. Kozarenko
Russian Federation
Olesya N. Kozarenko – MD, PhD, Researcher, Laboratory of Metagenomics and Food Biotechnology, Laboratory of Metagenomics and Food Biotechnology
Scopus Author ID: 57443919600
85 Pobedy Str., Belgorod 308015
19 Prospect Revolutsii, Voronezh 394036
O. B. Altukhova
Russian Federation
Oxana B. Altuhova – MD, Dr Med Sci, Associate Professor, Head of the Department of Obstetrics and Gynecology; Head of Gynecological Department
Scopus Author ID: 57216900558
85 Pobedy Str., Belgorod 308015
8/9 Nekrasova Str., Belgorod 308000
S. P. Pakhomov
Russian Federation
Sergey P. Pakhomov – MD, Dr Med Sci, Professor, Vice-Rector for Medical Activities and Regional Health Development; Professor, Department of Obstetrics and Gynecology
Scopus Author ID: 57211600515
3 Karl Marks Str., Kursk 305041
M. I. Churnosov
Russian Federation
Mikhail I. Churnosov – MD, Dr Med Sci, Professor, Head of the Department of Medical and Biological Disciplines
Scopus Author ID: 6601948788
85 Pobedy Str., Belgorod 308015
Review
For citations:
Lebedeva O.P., Ivannikova V.M., Zhukova I.O., Kozarenko O.N., Altukhova O.B., Pakhomov S.P., Churnosov M.I. NOD-like receptors in pathogenesis of missed and spontaneous abortions. Obstetrics, Gynecology and Reproduction. 2023;17(5):554-564. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.435

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































