Scroll to:
Clinical significance of ADAMTS-13/vWF axis in pregnant women at different trimesters of gestation
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.405
Abstract
Introduction. The pandemic of a novel coronavirus infection has demonstrated the importance of assessing the ADAMTS-13/vWF axis in patients with COVID-19, because a decline in this ratio mirrors disease severity. However very few data in the global literature on crosstalk and ADAMTS-13/vWF levels in pregnant women remaining very contradictory are available. Taking into consideration an impact of the ADAMTS-13/vWF axis on prevalence of thrombosis and disorders in the hemostasis system, investigation of this issue is highly demanded.
Aim: to assess the functioning of the ADAMTS-13/vWF axis during physiological pregnancy.
Materials and Methods. A controlled non-randomized study was conducted: main group included 44 women with physiologically occurring pregnancies at I, II and III trimesters; the control group consisted of 45 healthy non-pregnant women. The plasma level of ADAMTS-13 inhibitor (ADAMTS-13:i), ADAMTS-13 antigen (ADAMTS-13:Ag), vWF antigen (vWF:Ag), and ADAMTS-13 activity (ADAMTS-13:Ac) as well as relevant ratio (ADAMTS-13:Ac/vWF:Ag) were measured.
Results. It was shown that in parallel with increasing gestational age, significant changes occurred in the ADAMTS-13:Ac/vWF:Ag ratio. In main group, patients at II trimester were found to have level of ADAMTS-13:Ac/vWF:Ag 0.359 ± 0.121, in III trimester –0.253 ± 0.741, which significantly differed (p < 0.01) compared to control group with non-pregnant women (1,134 ± 0,308).
Conclusion. Our study provides new insights into the functioning of the ADAMTS-13:/vWF axis in women with physiologically occurring pregnancy at I, II and III trimesters. Decline in ADAMTS-13:Ac was demonstrated along with increasing vWF:Ag level observed in parallel with increasing gestational age. Apparently, the progressive decrease of ADAMTS-13 concentration during pregnancy is associated with its increased consumption due to high vWF level. However, due to the small single-center patient cohort, further studies with larger-scale studies are needed.
For citations:
Grigoreva K.N., Gashimova N.R., Bitsadze V.O., Pankratyeva L.L., Khizroeva J.Kh., Tretyakova M.V., Tsibizova V.I., Degtyareva N.D., Mulenkova A.V., Gris J., Kvaratskheliia M.V., Grandone E., Yakubova F.E., Blinov D.V., Makatsariya A.D. Clinical significance of ADAMTS-13/vWF axis in pregnant women at different trimesters of gestation. Obstetrics, Gynecology and Reproduction. 2023;17(2):221-230. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.405
Introduction / Введение
The hemostasis system being one of essential arms in hemostasis in health and disease of pregnancy has always been of great interest. Оwing to the advances in molecular biology occurred over the last decades, it was discovered that zinc-containing metalloproteinase ADAMTS-13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) and von Willebrand factor (vWF) play an extremely important role in hemostasis [1–3].
Von Willebrand factor is a plasma glycoprotein produced by the endothelium [4] exerting multiple functions in the human body primarily resulting in platelet recruitment and binding [5]. Microscopic studies showed that in the absence of endothelial damage, vWF in a folded form functions to preventing platelet adhesion. When a blood vessel becomes damaged, vWF “spreads out” and attracts ADAMTS-13 metalloproteinase that cleaves ultra-large vWF multimers [6]. Upon a large area of endothelial damage, a massive vWF release from Weibel–Palade bodies occurs leading to the so-called "relative" vWF-protease deficiency. ADAMTS-13 deficiency results in accumulated ultra-large vWF molecules and platelet adhesion followed by platelet consumption and microvasculature thrombosis [1][5].
Normal pregnancy is characterized by developing hypercoagulant state. In recent years, due to the COVID-19 pandemic, it has been found that endothelial damage in hyperinflammation occurs along with the ADAMTS-13/vWF system dysregulation [7] resulting in the microcirculatory disorders and leading to multiple organ failure. Preeclampsia (PE) is one of the prominent cases of the endotheliopathy developing during pregnancy. Patients with PE were found to have substantially altered pro- and anticoagulant pathways, which result in developing the super-hypercoagulable state [8][9].
Aim: to assess the functioning of the ADAMTS-13/vWF axis during physiological pregnancy.
Materials and Methods / Материалы и методы
Study design / Дизайн исследования
At the clinical facilities of the Perinatal Center of Vorokhobov City Clinical Hospital № 67 and "Medical Women's Center" LLC, there was conducted a controlled non-randomized study enrolling 193 patients – 148 pregnant and 45 non-pregnant women.
Inclusion and exclusion criteria / Критерии включения и исключения
Inclusion criteria for main group: age over 18 years; healthy pregnant women with a physiological pregnancy at the I, II and III trimesters; written voluntary informed consent to participate in the study
Inclusion criteria for control group: age over 18 years; no pregnancy confirmed; written voluntary informed consent to participate in the study.
Exclusion criteria: convalescent women who had acute respiratory infections (ARI) and bacterial infection during pregnancy; with PE history; PE developed during pregnancy; unsatisfactory results of the first screening; positive glucose tolerance test; confirmed positive test for antibodies against human immunodeficiency virus, markers of viral hepatitis B and C and syphilis; history of malignant neoplasms, somatic pathology; preterm delivery; refusal to participate in the study.
Study methods / Методы исследования
Peripheral blood samples (volume: 9 ml) were collected from pregnant females (main group) at three time points: at first screening (gestational age: from 11 to 13 weeks), during oral glucose tolerance test (gestational age: from 24 weeks to 26 weeks 6 days), and at first period of labor. In healthy non-pregnant women (control group), peri- pheral blood samples (volume: 9 ml) were collected at a single time point. Peripheral blood sampling was performed with a dry sterile needle from the cubital vein into a plastic tube added with an anticoagulant (sodium citrate solution 3.8 %), at 9:1 ratio. Whole blood was centrifuged at 2,500 g for 20 min at 20–24 °C followed by storage at –80 °C. Plasma level of ADAMTS-13 inhibitor (ADAMTS-13:i), ADAMTS-13 antigen (ADAMTS-13:Ag), vWF antigen (vWF:Ag) and ADAMTS-13 activity (ADAMTS-13:Ac) was assessed using commercially available test kits Technoclon Herstellung von Diagnostika und Arzneimitteln Gmb (TECHNOZYM®, Austria) followed by calculating the ADAMTS-13:Ac/vWF:Ag ratio. Normal refe- rence values were as follows: for ADAMTS-13:i < 15 U/ml, ADAMTS-13:Ag – 0.41–1.41 U/ml, vWF:Ag – 0.5–1.5 IU/ml (50–150 %) and activity ADAMTS-13:Ac – 0.4–1.3 IU/ml. Magnitude of the analyzed parameters in pregnant women at the I, II and III trimesters was compared with that of in non-pregnant women.
Ethical aspects / Этические аспекты
The study was approved by the Local Ethics Committee at the Sechenov University, Protocol No. 03-23 dated of February 16, 2023. All participants were informed about the study nature and the inclusion criteria as well as provided a signed voluntary informed consent and received relevant comprehensive information. The study was conducted in accordance with the ethical requirements of the Declaration of Helsinki of the World Medical Association
Statistical analysis / Статистический анализ
Statistical data processing retrieved from Microsoft Office Excel 2021 spreadsheets (Microsoft, USA) was performed using the Jamovi program, version 2.3.22 (The jamovi project, Australia). Statistical analysis included the calculation of descriptive statistics: mean (M), standard deviation (SD), median (Me), limit of the 95 % confidence interval (95 % CI). The Mann–Whitney U-test was used to compare inter-group parameters. Fisher's exact test was used to assess a significance for examined parameters: p ≤ 0.05 indicated the presence of significant differences, p > 0.05 – no differences.
Results and Discussion / Результаты и обсуждение
Patient groups / Группы обследованных
At the initial stage, there were enrolled 148 pregnant women. However, during the study, the following subjects were excluded: at trimester I – 7 pregnant women due to ARI and 11 due to unsatisfactory results of the first screening; at trimester II – 6 pregnant women due to ARI, 1 due to acute pyelonephritis and 4 due to a positive oral glucose tolerance test; at trimester III – 10 pregnant women due to ARI, 2 due to preterm delivery, 3 due to early PE, 19 due to late PE and 41 with physiological pregnancy due to delivery occurred in other medical facilities (Fig. 1).
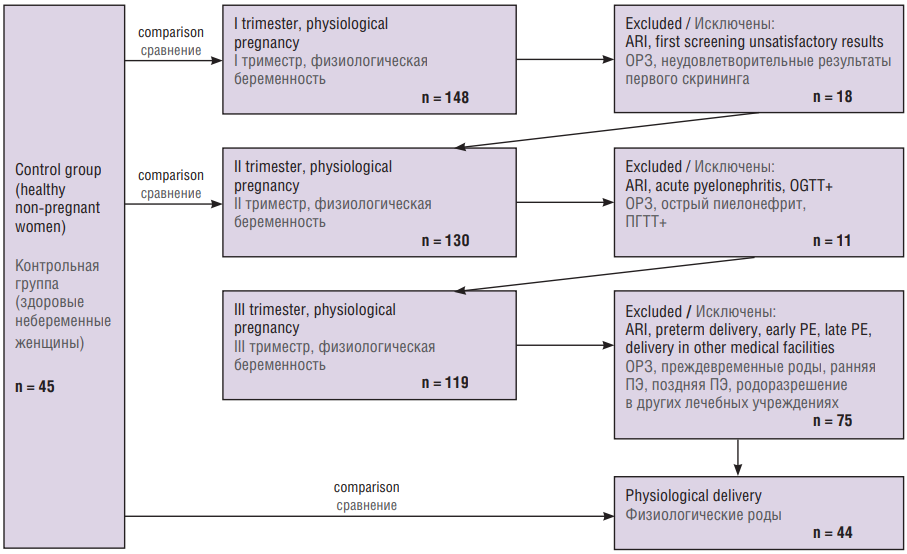
Figure 1. Patient selection flowchart.
Note: ARI – acute respiratory diseases; OGTT+ – positive oral glucose tolerance test, PE – preeclampsia
Рисунок 1. Схема отбора пациентов.
Примечание: ОРЗ – острые респираторные заболевания; ПГТТ+ – положительный пероральный глюкозотолерантный тест, ПЭ – преэклампсия.
The main group included 44 women with physiologically occurring pregnancies at the I, II and III trimesters, the control group – 45 healthy non-pregnant women planning pregnancy.
Clinical and anamnestic characteristics / Клинико-анамнестическая характеристика
Clinical and anamnestic examination data of study participants as well as perinatal outcomes are presented in Table 1.
Table 1. Clinical and anamnestic characteristics of the examined women and perinatal outcomes.
Таблица 1. Клинико-анамнестическая характеристика обследованных женщин и перинатальные исходы.
|
Parameter Показатель |
Main group Основная группа n = 44 |
Control group Контрольная группа n = 45 |
|
Age, years, M / Возраст, лет, М Min–max |
29,07 22–40 |
28,13 18–37 |
|
Body mass index, kg/m2, M / Индекс массы тела, кг/м2, М |
24,60 |
24,06 |
|
Blood group, n (%): / Группа крови, n (%): • blood group 0 / группа крови 0 • blood group А / группа крови А • blood group В / группа крови В • blood group АВ / группа крови АВ |
19 (43,19) 14 (31,81) 8 (18,19) 3 (6,81) |
15 (33,33) 16 (35,56) 10 (22,22) 4 (8,89) |
|
Obstetric and gynecological history, n: / Акушерско-гинекологический анамнез, n: • former pregnancies / беременности в анамнезе • term delivery / своевременные роды • preterm delivery / преждевременные роды • loss of pregnancy before 10 weeks / потери беременности до 10 нед • loss of pregnancy before 22 weeks / потери беременности до 22 нед |
19 15 1 6 1 |
16 15 0 4 0 |
|
Current pregnancy, n: / Настоящая беременность, n: • Natural delivery / роды через естественные родовые пути • Cesarian section / роды путем кесарева сечения • Apgar score at 1 min < 8 points / оценка по шкале Апгар на 1-й минуте < 8 баллов • Apgar score at 5 min < 8 points / оценка по шкале Апгар на 5-й минуте < 8 баллов |
33 11 1 0 |
– – – – |
The examined women did not differ in age, somatic status and body mass index, among them there were no cases of perinatal mortality. History of pregnancy loss before the week 10 in the main group was in 6 out of 44 (13.64 %), in the control group – in 4 out of 45 (8.89 %). A history of pregnancy loss before week 22 was observed only in the main group in 1 out of 44 (2.27%), its cause was premature rupture of the membranes. In the current pregnancy, vaginal delivery occurred in 33 out of 44 (75.0%) patients with physiological pregnancy at trimester III; delivery by caesarean section was performed in 11 of 44 (25.0 %), the delivery period ranged from 37 weeks to 41 weeks 2 days.
Laboratory results / Результаты лабораторных исследований
Table 2 presents the results of laboratory tests of the women examined.
Table 2. A panel of assessed laboratory parameters (M ± SD).
Таблица 2. Результаты изученных лабораторных показателей (М ± SD).
|
Parameter Параметр |
Main group / Основная группа |
Control group Контрольная группа n = 45 |
p |
||
|
I trimester I триместр n = 44 |
II trimester II триместр n = 44 |
III trimester III триместр n = 44 |
|||
|
ADAMTS-13:Aс, IU/ml ADAMTS-13:Aс, МЕ/мл |
0,846 ± 0,094 |
0,526 ± 0,112 |
0,446 ± 0,088 |
0,871 ± 0,109 |
0,534* < 0,01** < 0,01*** |
|
ADAMTS-13:Ag, U/ml ADAMTS-13:Ag, ЕД/мл |
0,991 ± 0,198 |
0,875 ± 0,099 |
0,773 ± 0,139 |
0,961 ± 0,212 |
0,270* 0,072** < 0,01*** |
|
ADAMTS-13:i, U/ml ADAMTS-13:i, ЕД/мл |
1,268 ± 0,697 |
1,417 ± 0,733 |
2,413 ± 1,659 |
1,486 ± 0,799 |
0,478* 0,906** 0,025*** |
|
vWF:Ag, IU/ml vWF:Ag, МЕ/мл |
0,945 ± 0,191 |
1,516 ± 0,122 |
1,761 ± 0,281 |
0,885 ± 0,222 |
0,110* < 0,01** < 0,01*** |
|
ADAMTS-13:Ac/vWF |
0,924 ± 0,323 |
0,359 ± 0,121 |
0,253 ± 0,741 |
1,134 ± 0,308 |
0,348* < 0,01** < 0,01*** |
Note: *significant differences between main group (I trimester) and control group; **significant differences between main group (II trimester) and control group, ***significant differences between main group (III trimester) and control group; ADAMTS-13:Ac – ADAMTS-13 activity; ADAMTS-13:Ag – ADAMTS-13 antigen; ADAMTS-13:I – ADAMTS-13 inhibitor; vWF:Ag – von Willebrand factor antigen; significant differences are highlighted in bold.
Примечание: *значимость различий между основной группой (I триместр) и контрольной группой; **значимость различий между основной группой (II триместр) и контрольной группой, ***значимость различий между основной группой (III триместр) и контрольной группой; ADAMTS-13:Aс – активность ADAMTS-13; ADAMTS-13:Ag – антиген ADAMTS-13; ADAMTS-13:i – ингибитор ADAMTS-13; vWF:Ag – антиген фактора фон Виллебранда; выделены значимые различия.
The examined pregnant women showed statistical differences in ADAMTS-13:Ac magnitude at trimesters II and III compared with control group (p < 0.01) indicating that, normally, along with higher gestation period, ADAMTS-13 activity gradually declines. At the same time, at trimester I, it almost matched the level in non-pregnant women (Fig. 2).
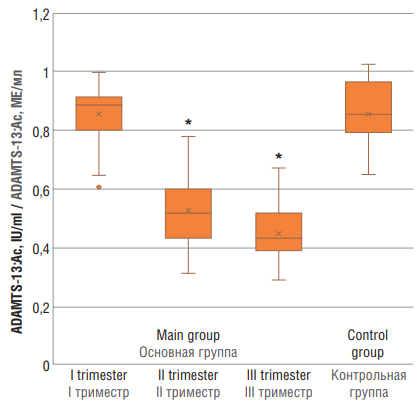
Figure 2. ADAMTS-13 (ADAMTS-13:Ac) activity in women with physiological pregnancy at I, II and III trimesters (main group) and in healthy non-pregnant women (control group).
Note: *p < 0.01 – significant differences compared to control group.
Рисунок 2. Уровень активности ADAMTS-13 (ADAMTS-13:Aс) у женщин с физиологически протекающей беременностью в I, II и III триместрах (основная группа) и у здоровых небеременных женщин (контрольная группа).
Примечание: *р < 0,01 – различия статистически значимы по сравнению с контрольной группой.
While assessing ADAMTS-13:Ag (Fig. 3), significant differences were observed solely between main group at trimester III and the control group (p < 0.01).
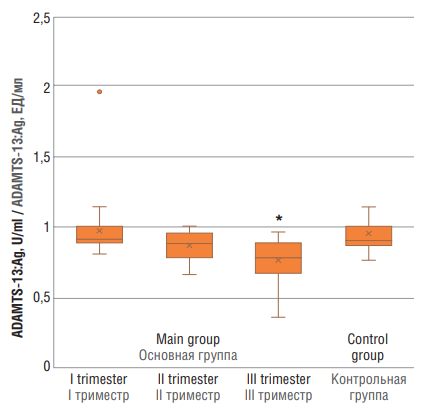
Figure 3. ADAMTS-13 antigen (ADAMTS-13:Ag) level in women with physiological pregnancy at I, II and III trimesters (main group and healthy non-pregnant women (control group).
Note: *p < 0.01 – significant differences compared to control group.
Рисунок 3. Уровень антигена ADAMTS-13 (ADAMTS-13:Ag) у женщин с физиологически протекающей беременностью в I, II и III триместрах (основная группа) и у здоровых небеременных женщин (контрольная группа).
Примечание: *р < 0,01 – различия статистически значимы по сравнению с контрольной группой.
ADAMTS-13:i values in main group were as follows: at trimester I – 1.268 ± 0.697 U/ml, trimester II – 1.417 ± 0.733 U/ml, and trimester III – 2.413 ± 1.659 U/ml. ADAMTS-13:i values in main group at trimester III vs. control group (1.486 ± 0.799 U/ml) differed significantly (p = 0.025), whereas ADAMTS-13:i magnitude was within the reference range (Fig. 4 ).
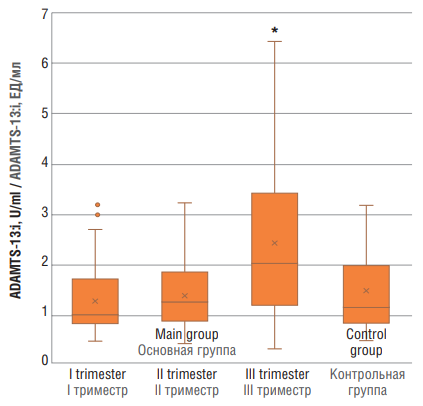
Figure 4. ADAMTS-13 inhibitor (ADAMTS-13:i) level in women with physiological pregnancy at I, II and III trimesters (main group) and in healthy non-pregnant women (control group).
Note: *p < 0.05 – significant differences compared to control group.
Рисунок 4. Уровень ингибитора ADAMTS-13 (ADAMTS-13:i) у женщин с физиологически протекающей беременностью в I, II и III триместрах (основная группа) и у здоровых небеременных женщин (контрольная группа).
Примечание: *р < 0,05 – различия статистически значимы по сравнению с контрольной группой.
Significant differences in vWF:Ag level were noted between pregnant women in main group at trimesters II and III (p < 0.01), but not at trimester I vs. control group (Fig. 5).
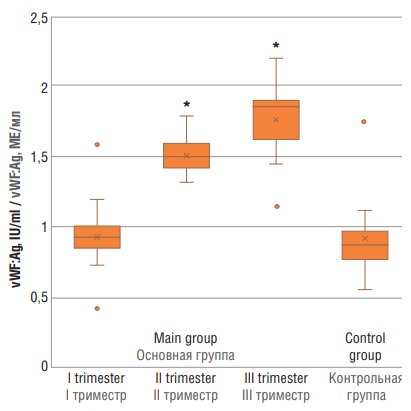
Figure 5. von Willebrand factor antigen (vWF:Ag) level in women with physiological pregnancy at I, II and III trimesters (main group) and in healthy non-pregnant women (control group).
Note: *p < 0.01 – significant differences compared to control group.
Рисунок 5. Уровень антигена фактора фон Виллебранда (vWF:Ag) у женщин с физиологически протекающей беременностью в I, II и III триместрах (основная группа) и у здоровых небеременных женщин (контрольная группа).
Примечание:*р < 0,01 – различия статистически значимы по сравнению с контрольной группой.
As shown by many studies, vWF tends to increase in parallel with gestational age [10][11]. Other studies showing that vWF levels markedly increase in patients with PE compared with physiological pregnancy at trimester III [12][13]. However, some researchers reject such data and demonstrate no differences between these groups of patients [14]. Regarding ADAMTS-13, it is much more complicated; no global consensus about altered ADAMTS-13 activity during pregnancy was reached. Some studies demonstrate that its decline begins from trimester II of physiological pregnancy [15][16]; others state that it remains unaltered compared with that in physiological pregnancy and non-pregnant women [17]. Our study clearly demonstrates that along with increasing gestation age, ADAMTS-13 activity decreases, whereas vWF level steadily elevates. Most likely, it is due to the massive vWF release resulting in its consumption.
Assessing ADAMTS-13:Aс/vWF:Ag axis revealed significant differences (p < 0.01) between main group at trimesters II and III vs. control group (Fig. 6).
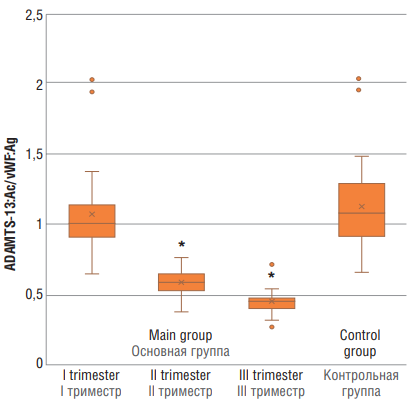
Figure 6. ADAMTS-13:Ac/vWF:Ag ratio in women with physiological pregnancy at I, II and III trimesters (main group) and in healthy non-pregnant women (control group).
Note: *p < 0.01 – significant differences compared to control group.
Рисунок 6. Отношение ADAMTS-13:Aс/vWF:Ag у женщин с физиологически протекающей беременностью в I, II и III триместрах (основная группа) и у здоровых небеременных женщин (контрольная группа).
Примечание:*р < 0,01 – различия статистически значимы по сравнению с контрольной группой.
A decline in the ADAMTS-13:Ac/vWF:Ag ratio in COVID-19 demonstrated by numerous studies evidence about development of progressive endotheliopathy and several times precipitates a risk of thrombosis including thrombotic microangiopathy [18–20].
Conclusion / Заключение
Our study provides a novel insight into ADAMTS-13:Ac/vWF:Ag axis in women with normal pregnancies at trimesters I, II, III. The COVID-19 pandemic uncovered that decline in the ADAMTS-13:Ac/vWF:Ag axis was one of the most important severity predictors that also resulted in elevated risk of thrombotic complications, being the main marker of endotheliopathy. Moreover, it was also elucidated that higher gestation age is paralleled with lowered ADAMTS-13:Aс level along with rise in vWF:Ag that might evidence about developing endotheliopathy. However, due to the small single-center sample, further studies with a larger patient cohorts are required.
References
1. Moake J.L., Rudy C.K., Troll J.H. et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;(23)307:1432–5. https://doi.org/10.1056/NEJM198212023072306.
2. Tsai H.M. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87(10):4235–44.
3. Furlan M., Robles R., Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–34.
4. Zhou Y.-F., Eng E.T., Zhu J. et al. Sequence and structure relationships within von Willebrand factor. Blood. 2012;120(2):449–58. https://doi.org/10.1182/blood-2012-01-405134.
5. Lenting P.J., Christophe O.D., Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125(13):2019–28. https://doi.org/10.1182/blood-2014-06-528406.
6. De Ceunynck K., De Meyer S.F., Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121(2):270–7. https://doi.org/10.1182/blood-2012-07-442285.
7. Grandone E., Vimercati A., Sorrentino F. et al. Obstetric outcomes in pregnant COVID-19 women: the imbalance of von Willebrand factor and ADAMTS13 axis. BMC Pregnancy Childbirth. 2022;22(1):142. https://doi.org/10.1186/s12884-022-04405-8.
8. English F.A., Kenny L.C., McCarthy F.P. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015;8:7–12. https://doi.org/10.2147/IBPC.S50641.
9. Gris J.-C., Bouvier S., Cochery-Nouvellon E. et al. The role of haemostasis in placenta-mediated complications. Thromb Res. 2019;181 Suppl 1:S10–S14. https://doi.org/10.1016/S0049-3848(19)30359-7.
10. Drury-Stewart D., Lannert K., Chung D. et al. Complex changes in von Willebrand factor-associated parameters are acquired during uncomplicated pregnancy. PLoS One. 2014;9(11):e112935. https://doi.org/10.1371/journal.pone.0112935.
11. Chen Y., Huang P., Han C. et al. Association of placenta-derived extracellular vesicles with pre-eclampsia and associated hypercoagulability: A clinical observational study. BJOG. 2020;128(6):1037–46. https://doi.org/10.1111/1471-0528.16552.
12. Gadisseur A., Berneman Z., Schroyens W., Michiels J.J. Laboratory diagnosis of von Willebrand disease type 1/2E (2A subtype IIE), type 1 Vicenza and mild type 1 caused by mutations in the D3, D4, B1-B3 and C1-C2 domains of the von Willebrand factor gene. Role of von Willebrand factor multimers and the von Willebrand factor propeptide/antigen ratio. Acta Haematol. 2009;121(2–3):128–38. https://doi.org/10.1159/000214853.
13. Hulstein J., Heimel P., Franx A. et al. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J Thromb Haemost. 2006;4(12):2569–75. https://doi.org/10.1111/j.1538-7836.2006.02205.x.
14. Stepanian A., Cohen-Moatti M., Sanglier T. et al. Von Willebrand factor and ADAMTS13: a candidate couple for preeclampsia pathophysiology. Arterioscler Thromb Vasc Biol. 2011;31(7):1703–9. https://doi.org/10.1161/ATVBAHA.111.223610.
15. Ramadan M., Badr D., Hubeish M. et al. HELLP syndrome, thrombotic thrombocytopenic purpura or both: appraising the complex association and proposing a stepwise practical plan for differential diagnosis. J Hematol. 2018;7(1):32–7. https://doi.org/10.14740/jh347w.
16. Sánchez-Luceros A., Farías C., Amaral M. et al. von Willebrand factor-cleaving protease (ADAMTS13) activity in normal non-pregnant women, pregnant and post-delivery women. Thromb Haemost. 2004;92(6):1320–6. https://doi.org/10.1160/TH03-11-0683.
17. Molvarec A., Rigó J., Bõze T. et al. Increased plasma von Willebrand factor antigen levels but normal von Willebrand factor cleaving protease (ADAMTS13) activity in preeclampsia. Thromb Haemost. 2009;101(2):305–11.
18. Joly B.S., Darmon M., Dekimpe C. et al. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19 patients. J Thromb Haemost. 2021;19(9):2193–8. https://doi.org/10.1111/jth.15445.
19. Favaloro E.J., Henry B.M., Lippi G. Increased VWF and decreased ADAMTS-13 in COVID-19: creating a milieu for (micro)thrombosis. Semin Thromb Hemost. 2021;47(4):400–18. https://doi.org/10.1055/s-0041-1727282.
20. Doevelaar A., Bachmann M., Hölzer B. et al. COVID-19 is associated with relative ADAMTS13 deficiency and VWF multimer formation resembling TTP (Preprint). medRxiv. August 25, 2020. https://doi.org/10.1101/2020.0.
About the Authors
K. N. GrigorevaRussian Federation
Kristina N. Grigoreva – MD, Assistant, Department of Obstetrics, Gynecology and Perinatal Medicine
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
N. R. Gashimova
Russian Federation
Nilufar R. Gashimova – MD, Postgraduate Student, Department of Obstetrics, Gynecology and Perinatal Medicine
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
V. O. Bitsadze
Russian Federation
Victoria O. Bitsadze – MD, Dr Sci Med, Professor of RAS, Professor, Department of Obstetrics, Gynecology and Perinatal Medicine
Scopus Author ID: 6506003478, Researcher ID: F-8409-
2017
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
L. L. Pankratyeva
Russian Federation
Liudmila L. Pankratyeva – MD, Dr Sci Med, Associate Professor, Professor, Department of Pediatrics and Health Organization, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology; Neonatologist, Hematologist, Head of the Clinical Research Center, Vorokhobov City Clinical Hospital No 67
1 Samora Machel Str., Moscow 117997
2/44 Salyama Adilya Str., Moscow 123423
J. Kh. Khizroeva
Russian Federation
Jamilya Kh. Khizroeva – MD, Dr Sci Med, Professor, Department of Obstetrics, Gynecology and Perinatal Medicine
Scopus Author ID: 57194547147, Researcher ID: F-8384-2017
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
M. V. Tretyakova
Russian Federation
Maria V. Tretyakova – MD, PhD, Obstetrician-Gynecologist, Assistant, Department of Obstetrics, Gynecology and Perinatal Medicine
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
V. I. Tsibizova
Russian Federation
Valentina I. Tsibizova – MD, PhD, Obstetrician-Gynecologist, Research Laboratory of Operative Gynecology, Institute of Perinatology and Pediatrics; Physician, Department of Functional and Ultrasound Diagnostics
2 Akkuratova Str., Saint Petersburg 197341
N. D. Degtyareva
Russian Federation
Natalia D. Degtyareva – Student
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
A. V. Mulenkova
Russian Federation
Alyona V. Mulenkova – Student
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
J.-C. Gris
France
Jean-Christophe Gris – MD, Dr Sci Med, Professor, Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health,
Sechenov University; Professor of Haematology, Head of the Laboratory of Haematology, Faculty of Biological and Pharmaceutical Sciences, Montpellier University and University Hospital of Nîmes, France; Foreign Member of RAS
Scopus Author ID: 7005114260, Researcher ID: AAA-2923-2019
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
163 Rue Auguste Broussonnet, Montpellier 34090, France
M. V. Kvaratskheliia
Russian Federation
Margaret V. Kvaratskheliia – MD, Medical Resident, Department of Obstetrics, Gynecology and Perinatal Medicine
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
E. Grandone
Russian Federation
Elvira Grandone – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health, Sechenov University; Head of the Department of Thrombosis and Hemostasis, Research Center «Casa Sollievo della Sofferenza», San Giovanni Rotondo, Italia
Scopus Author ID: 7006391091, Researcher ID: M-1127-2019
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
1 Viale Cappuccini, San Giovanni Rotondo 71013, Italia
F. E. Yakubova
Russian Federation
Fidan E. Yakubova – MD, Clinical Resident, Department of Obstetrics and Gynecology
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
D. V. Blinov
Russian Federation
Dmitry V. Blinov – MD, PhD, MBA, Assistant, Department of Sports Medicine and Medical Rehabilitation, Sklifosovsky Institute of Clinical Medicine, Sechenov
University; Head of Medical and Scientific Affairs, Institute for Preventive and Social Medicine; Associate Professor, Department of Sports, Physical and Rehabilitation Medicine, Moscow Haass Medical – Social Institute
Scopus Author ID: 6701744871, Researcher ID: E-8906-2017, RSCI: 9779-8290
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
4–10 Sadovaya-Triumfalnaya Str., Moscow 127006
5 bldg. 1–1a, 2-ya Brestskaya Str., Moscow 123056
A. D. Makatsariya
Russian Federation
Alexander D. Makatsariya – MD, Dr Sci Med, Academician of RAS, Professor, Head of the Department of Obstetrics, Gynecology and Perinatal Medicine, Filatov Clinical Institute of Children’s Health, Sechenov University; Vice-President of the Russian Society of Obstetrican-Gynecologists (RSOG); Honorary Doctor of the Russian Federation; Emeritus Professor of the University of Vienna
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Review
For citations:
Grigoreva K.N., Gashimova N.R., Bitsadze V.O., Pankratyeva L.L., Khizroeva J.Kh., Tretyakova M.V., Tsibizova V.I., Degtyareva N.D., Mulenkova A.V., Gris J., Kvaratskheliia M.V., Grandone E., Yakubova F.E., Blinov D.V., Makatsariya A.D. Clinical significance of ADAMTS-13/vWF axis in pregnant women at different trimesters of gestation. Obstetrics, Gynecology and Reproduction. 2023;17(2):221-230. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2023.405

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































