Scroll to:
Neutrophil extracellular traps as markers of thromboinflammation in the pathogenesis of female genital tract and breast malignant neoplasms
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.335
Abstract
Introduction. Thrombosis is a formidable complication of the oncological process that still profoundly contributes to overall mortality despite the anticoagulant use. According to the recent data, thrombosis in cancer represents a special type of prothrombotic state, wherein thromboinflammationis one of its constituents. In addition, thromboinflammation contributes both to the disease progression and intensity of metastasis processes. Recently, a large number of studies worldwide have been devoted to investigating thromboinflammation in cancer patients.
Aim: to assess NETosis activity (the process of neutrophils extracellular traps synthesis), namely, the concentration of citrullinated histone H3 (citH3) as a blood plasma NETosis marker in women with malignant genital organs and breast neoplasms.
Materials and Мethods. The prospective observational controlled non-randomized study included 45 patients (main group) with malignant neoplasms of uterus body, ovaries, cervix (adenocarcinoma of the cervical canal) and mammary glands admitted to the hospital for planned surgical treatment (13, 15, 5 and 13 patients, respectively) and 33 women with benign neoplasms of the genital organs and mammary gland (control group). The plasma citН3 concentration was determined using an enzyme immunoassay.
Results. It was found that cancer patients had citН3 concentration significantly increased (1.434–2.058 ng/ml) compared with the control group (0.281–0.371 ng/ml). The concentration of citH3 in patients with tumors of the uterine body and cervix ranged from 2.271 to 2.992 ng/ml, patients with ovarian tumors – from 1.357 to 2.123 ng/ml, patients with breast tumors – from 0.331 to 0.859 ng/ml. The study revealed no significant differences in the citH3 concentration in patients with breast tumors compared to the control group. Upon elevating citН3 concentration, such parameters as C-reactive protein, D-dimer, neutrophils and platelets count, as well as neutrophils/lymphocytes ratio were significantly increased. Significant differences were revealed in platelet count in the main group (236,68–273,77×109/L) vs. control group (178,14–202,35×109/L).
Conclusion. The study demonstrated the activation of NETosis in patients with tumors of the uterine body as well as cervix (adenocarcinoma) and ovaries that might be combined with hemostasis activation and systemic inflammatory response.
Keywords
For citations:
Slukhanchuk E.V., Bitsadze V.O., Solopova A.G., Khizroeva J.Kh., Gris J., Elalamy I., Makatsariya A.D. Neutrophil extracellular traps as markers of thromboinflammation in the pathogenesis of female genital tract and breast malignant neoplasms. Obstetrics, Gynecology and Reproduction. 2022;16(4):426-437. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.335
Introduction / Введение
Neutrophils along with dendritic cells and macrophages similar to phagocytes are essential components of innate immunity [1]. While involving diverse mechanisms [2] in own arsenal, they reflect the threat posed by various pathogens, including bacteria, fungi, protozoa, viruses, antibodies, immune complexes, cytokines, and chemokines such as interleukin-8 (IL-8), tumor necrosis factor (TNF), etc. Neutrophils undergo a specialized process of programmed death (without caspase activation during apoptosis), which leads to chromatin decondensation with the network formation called neutrophil extracellular traps (NETs).
Neutrophil extracellular traps were first identified in 2004 [3][4]. NETs are composed of strands of deconcentrated DNA and various protein/enzyme components as well as chemicals, including myeloperoxidase (MPO), neutrophil elastase, and tissue factor [5]. Myeloperoxidase is a peroxidase that uses a heme moiety as a cofactor and produces hypochlorous acid (HOCl) from hydrogen peroxide (H2O2) and chloride anion (Cl–) during the neutrophil oxidative burst [6]. Neutrophil elastase hydrolyzes proteins within specialized neutrophil lysosomes known as azurophilic granules and degrades outer membrane protein A as well as bacterial virulence factors [7]. In human tissues, neutrophil elastase in NETs can also degrade the extracellular matrix components, including collagen IV and elastin.
One of the critical steps in NETs formation is histone citrullination, carried out by the histone-specific enzyme peptidyl arginine deiminase 4 (PAD4). New data indicate that citrullinated histone H3 (citH3), a key component of NETs, can be recognized as a specific marker of NETosis [8] in both tissue samples and peripheral blood [9]. Histone hypercitrullination is an essential step in developing NETs, and citH3 is considered as a new and specific biomarker for this process. The destructive effect of NETs against pathogens is accounted for by their oxidation due to MPO and HOCl as well as cleavage by neutrophil elastase. Powerful polyvalent effects of NETs are not specific; dysregulated NETosis events during acute and chronic inflammation leads to tissue damage, fibrosis, hypercoagulability, thrombosis, atherosclerosis, tumor progression, and metastasis [5][10–12]. The formation of NETs results in local thrombosis, apparently aimed initially at preventing spread of pathogens. In addition, NETs have been shown to increase the intensity of fibrosis in various tissues, particularly in the lungs. Moreover, it was shown that pulmonary fibrosis is induced by NETs and dependent on PAD4 [13].
One of the most common complications and significant causes of death in cancer patients is a cancer-associated thrombosis. The risk of primary venous thromboembolism (VTE) in cancer is increased by 4–7 times, recurrent VTE – by 3 times, anticoagulantassociated bleeding – by 2 times, VTE-related death – by 10–7 times, and arterial thromboembolism – by 2-fold compared with non-cancer patients [14][15]. Risk factors for cancer-related thrombosis may be related to patient characteristics (ethnicity, age, comorbidities, etc.), tumor parameters (histological type, grade, primary site and post-diagnosis time frame, etc.), treatment strategy (chemotherapy, surgery/hospitalization, anti-angiogenic drugs, central venous catheters, erythropoietin stimulators, etc.), as well as essential biomarkers (leukocyte and platelet counts, D-dimer, hemoglobin, etc.) [16]. Recent studies have shown that NETs exert multivalent procoagulant properties, providing a "scaffold" support for procoagulant molecules in association with erythrocytes and platelets, promoting thrombosis in vitro and in vivo. NETs contain complex protein components that can activate the endogenous coagulation pathway promoting thrombus formation. Thus, it has been proven that NETs are essential participants in thrombogenesis in cancer patients [17][18].
Currently, a role of NETs in the initiation, formation, cancer metastasis and cancer-related thrombosis has been actively investigated. It turned out that tumor can induce NETosis while creating a hypoxic microenvironment due to higher expression of the transcription factor HIF-1α (hypoxia-inducible factor-Iα) and reactive oxygen species in severe oxidative stress. Tumor-secreted cytokines, proteases, and exosomes also trigger NETosis [19]. The hypoxic microenvironment and oxidative stress, which promote transforming growth factor-β1 (TGF-β1) secretion are the key inducers of the endothelial-mesenchymal transition (EMT). TGF-β plays prominent role in the EMT process in tumor microenvironment during oncogenesis and metastasis spread. NETs have been shown to stimulate EMT to facilitate oncogenesis [20][21], tumor growth [22], and metastasis [22][23]. Therefore, NETs can promote tumor growth and progression by accumulating in the tumor microenvironment [24]. NETs promote long-distance tumor spread by protecting tumor cells and preventing attack by circulating cytotoxic lymphocytes [25]. NETbound neutrophil elastase, matrix metalloproteinase 9, and cathepsin G disrupt the intercellular junctions and basement membrane via VE-cadherin proteolysis, thereby increasing endothelial cell permeability. This process also activates endothelial cells to recruit circulating tumor cells captured by NETs to promote cancer metastasis [21].
The first study examined NETs in tumor tissue obtained from patients with Ewing's sarcoma. Tumor-associated NETs have been identified in patients with poor prognosis [26]. In addition, NETs have been found in colon tumor tissues and metastatic lymph nodes. NETs level gradually declines from the center of the tumor to its distal edge [27]. Moreover, the NETs concentration in lymphoma tissue samples positively correlated with relevant level in blood plasma. High levels of NETs correlated with poor survival. Another study showed that elevated level of circulating NETs in the postoperative period of patients undergoing liver resection for metastatic colorectal cancer was associated with reduced disease-free survival [28]. In patients with primary liver carcinoma associated with non-alcoholic steatohepatitis, the NETs concentration was higher than in those with healthy liver or benign liver disease [29].
Aim: to assess NETosis activity (the process of neutrophil extracellular traps formation), by assessing blood plasma citH3 concentration as NETosis marker in women with malignant genital organ and breast neoplasms.
Materials and Methods / Материалы и методы
Study design / Дизайн исследования
A prospective controlled observational nonrandomized study was conducted from September, 2019 to March, 2022, at the Moscow City Clinical Oncological Hospital № 1 as well as at Petrovsky National Research Centre of Surgery that enrolled 78 patients, aged 30 to 72 years, hospitalized for planned surgical treatment.
Groups of patients examined / Группы обследованных пациенток
The main group included 45 patients with malignant genital organ and mammary gland neoplasms, stages I– III: ovarian cancer (n = 13), cancer of the uterine body (n = 15), cervical cancer (adenocarcinoma of the cervical canal, n = 5) and breast cancer (n = 13).
The control group consisted of 33 women with benign female genital organ and breast neoplasms.
Inclusion and exclusion criteria / Критерии включения и исключения
Inclusion criteria for the main group: the presence of a malignant neoplasm of the ovaries, uterine body, cervix and mammary glands, confirmed by clinical and instrumental examination; voluntary informed consent to participate in the study.
Inclusion criteria for the control group: verified benign female genital organ and breast neoplasms; no active cancer and oncological diseases, thrombosis and thromboembolism, chronic inflammatory diseases in history; voluntary informed consent to participate in the study.
Exclusion criteria: verified active infectious and/or inflammatory process; allergic reaction to contrast agents; use of anticoagulants, chemotherapy drugs; signs of thrombotic or hemorrhagic syndrome at first examination.
Study methods / Методы исследования
One day before surgery, fasting blood samples were collected by using dry sterile needle from the cubital vein into a plastic tube in 9:1 blood:anticoagulant ratio. A 3.8 % solution of trisubstituted sodium citrate was used as an anticoagulant.
Histone hypercitrullination is an important step in NETs formation, and citH3 is considered as a new and specific biomarker for this process. Assessing blood plasma citH3 level in patients of the main and control groups was carried out using enzyme-linked immunosorbent assay (Citrullinated Histone H3 ELISA Kit, Cayman Chemical, Ann Arbor, Michigan, USA).
D-dimer concentration was measured using the Dimertest latex test (Agen, Australia), based on using latex particle-coupled highly specific D-dimer antibody. Assessing D-dimer level is one of the most specific assays to diagnose DIC, thrombophilia and thrombotic complications, allowing to reveal intensity of fibrin polymerization during intravascular blood coagulation.
Complete blood count and C-reactive protein (CRP) level in venous blood samples were assessed using routine techniques.
Ethical aspects / Этические аспекты
The study was conducted in accordance with the requirements of the Declaration of Helsinki of the World Medical Organization. All patients received comprehensive information and signed up an informed consent.
Statistical analysis / Статистический анализ
Statistical analysis was performed in IBM SPSS Statistics version 25 software using nonparametric Mann–Whitney, Kruskal–Wallis tests, confidence interval analysis, and Dunnett's posterior test. Significant differences were assessed in case of reaching a significance level set at p < 0.05, as well as in case of differences between confidence limits in the groups compared. A correlation-regression analysis was used to analyze a relationship between quantitative variables. At the stage of correlation analysis, the nonparametric Spearman test was used (the relationship is considered weak when a correlation coefficient is up to 0.3, medium – from 0.3 to 0.7, and high – above 0.7). The use of nonparametric statistics is related to the lack of normality in variables distribution.
Results / Результаты
Clinical and anamnestic characteristics of patients examined / Клинико-анамнестическая характеристика обследованных пациенток
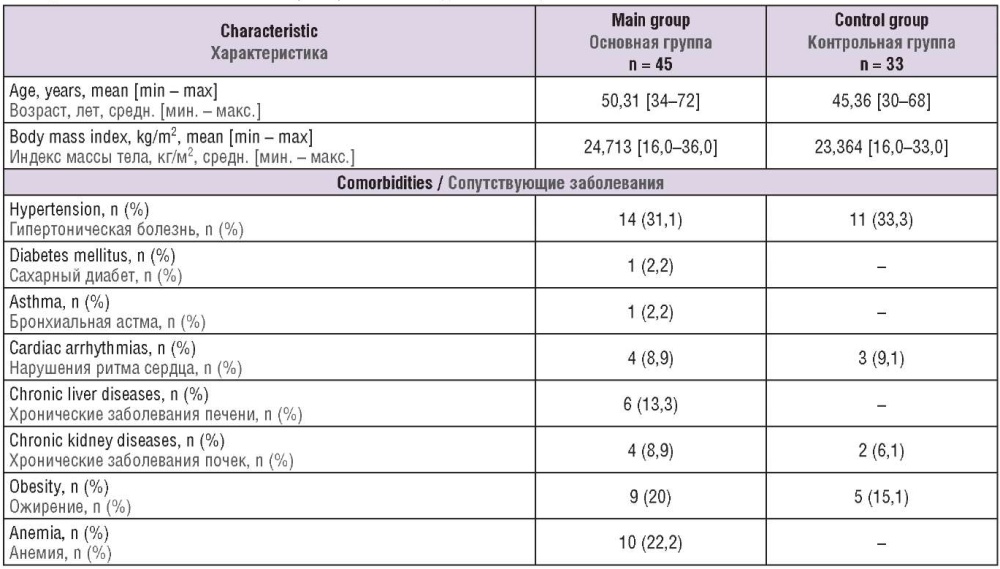
Clinical and anamnestic data of the women examined are presented in Table 1. Patients of the main and control groups did not significantly differ in age and body mass index. Noteworthy, a large number of obese patients were observed in both main and control groups, especially among women with benign and malignant breast and endometrial diseases. In the main group, the number of patients with anemia was markedly increased, especially in the subgroups of endometrial and ovarian cancer.
Table 1. Clinical and anamnestic characteristics of women examined.
Таблица 1. Клинико-анамнестическая характеристика обследованных пациенток.
The data on assessing the studied laboratory parameters in patients from both groups are shown Table 2. Significant differences (p < 0.05) between main and control group were revealed in such parameters as D-dimer (1.635 μg/ml vs. 0.379 μg/ml) and citH3 (1.746 ng/ml vs. 0.326 ng/ml) level, the neutrophil/lymphocyte ratio (6.1 vs. 1.914), platelet count (255.2×109/L vs. 190.2×109/L) and C-reactive protein concentration (3.779 mg/dL vs. 0.207 mg/dL), respectively.
Table 2. Laboratory blood parameters of patients examined.
Таблица 2. Лабораторные показатели крови обследованных пациенток.
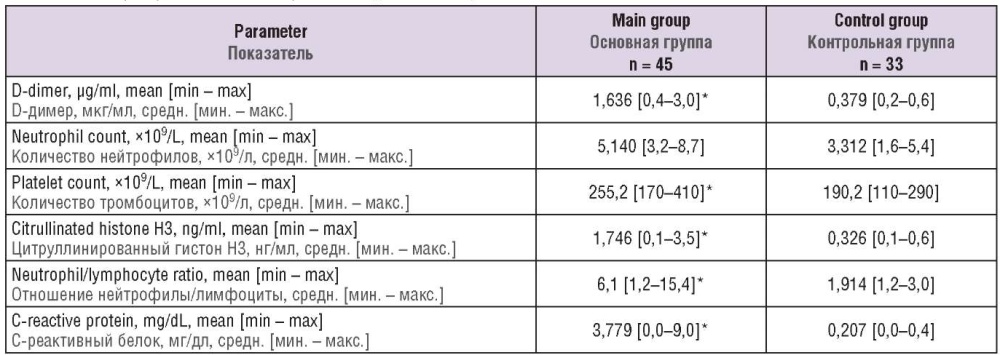
Note: *p < 0.05 – significant differences compared to control group.
Примечание: *р < 0,05 – различия статистически значимы по сравнению с контрольной группой.
The nonparametric Mann–Whitney test was used to search for differences in citH3 concentrations in main and control groups that allowed to find a high significance of differences (p = 0.0001). Using a confidence interval allowed to detect that 95 % of oncological vs. control subjects had citH3 level ranged from 1.434 to 2.058 ng/ml vs. 0.281 to 0.371 ng/ml (Fig. 1).
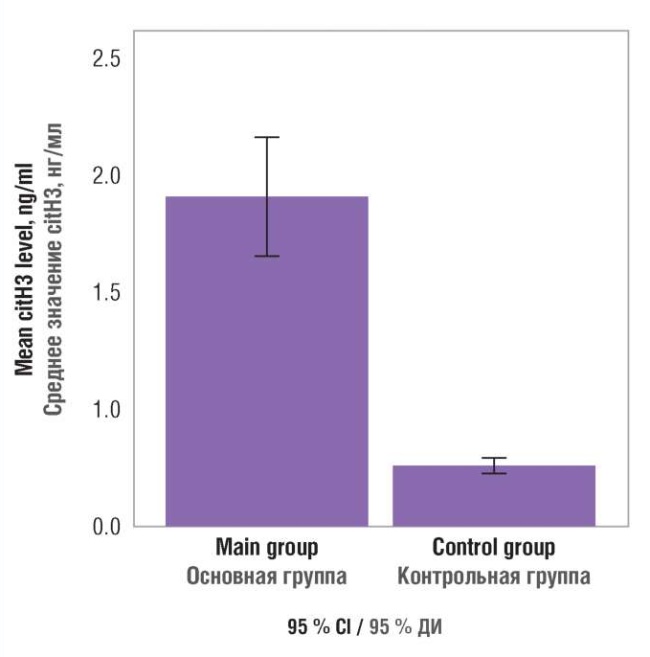
Figure 1. Blood plasma citrullinated histone H3 (citH3) level in patients examined.
Рисунок 1. Содержание цитруллинированного гистона Н3 (citH3) в плазме крови обследованных пациенток.
Nonparametric Kruskal–Wallis test, confidence interval analysis, and Dunnett's posterior test were used to detect differences in citH3 concentration among cancer patients with different tumor sources. It was found that citH3 concentration in patients with uterine body and cervical tumor ranged from 2.271 to 2.992 ng/ml, ovarian tumors – from 1.357 to 2.123 ng/ml, and breast tumors – from 0.331 to 0.859 ng/ml. Thus, serum citH3 concentration in patients with breast tumors did not significantly differ from control group where it ranged from 0.281 to 0.371 ng/ml (Fig. 2, Table 3).
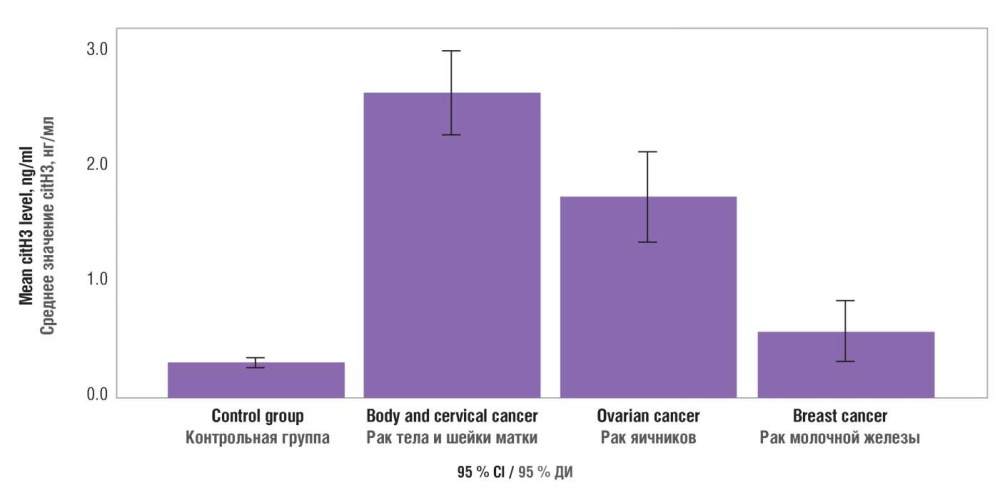
Figure 2. Blood plasma citrullinated histone H3 (citH3) level in varying tumor tissue sources.
Рисунок 2. Содержание цитруллинированного гистона Н3 (citH3) в плазме крови при различных источниках опухоли.
Table 3. Blood plasma citrullinated histone H3 (citH3) level in varying tumor tissue sources.
Таблица 3. Содержание цитруллинированного гистона Н3 (citH3) в плазме крови при различных источниках опухоли.
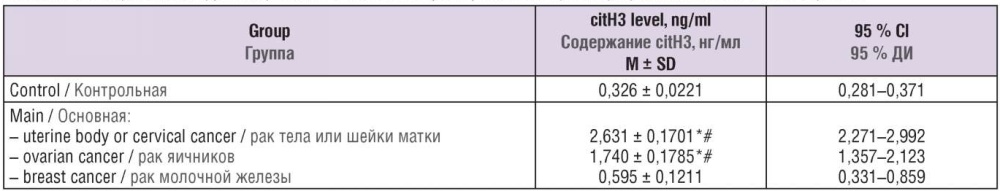
Note: *p < 0.05 – significant differences compared to control group; #p < 0.05 – significant differences compared to patients with breast cancer; M – mean; SD – standard deviation; 95 % Cl – 95 % confidence interval.
Примечание: *р < 0,05 – различия статистически значимы по сравнению с контрольной группой; #р < 0,05 – различия статистически значимы по сравнению с пациентами с раком молочной железы; М – среднее значение; SD – стандартное отклонение; 95 % ДИ – 95 % доверительный интервал.
A relationship between the concentration of citH3, CRP, D-dimer as a marker of hemostasis activation, neutrophil and platelet count, and neutrophil/lymphocyte ratio in cancer patients and patients in control group was assessed using correlation-regression analysis and Spearman's nonparametric test. As a result of the analysis, a significant (p = 0.0001) degree of correlation between all independent variables (CRP, D-dimer, neutrophils, platelets, neutrophil/lymphocyte ratio) and citH3 level was found out. The correlation coefficient reached an average level (from 0.3 to 0.7), except for citH3/CRP correlation that peaked reaching 0.75. citH3 magnitude increases almost linearly up to the level of 2 ng/ml paralleled with rise in CRP level from 5 to 6 mg/dL (Fig. 3). Further, an increase in citH3 concentration is not accompanied by increased CRP level.
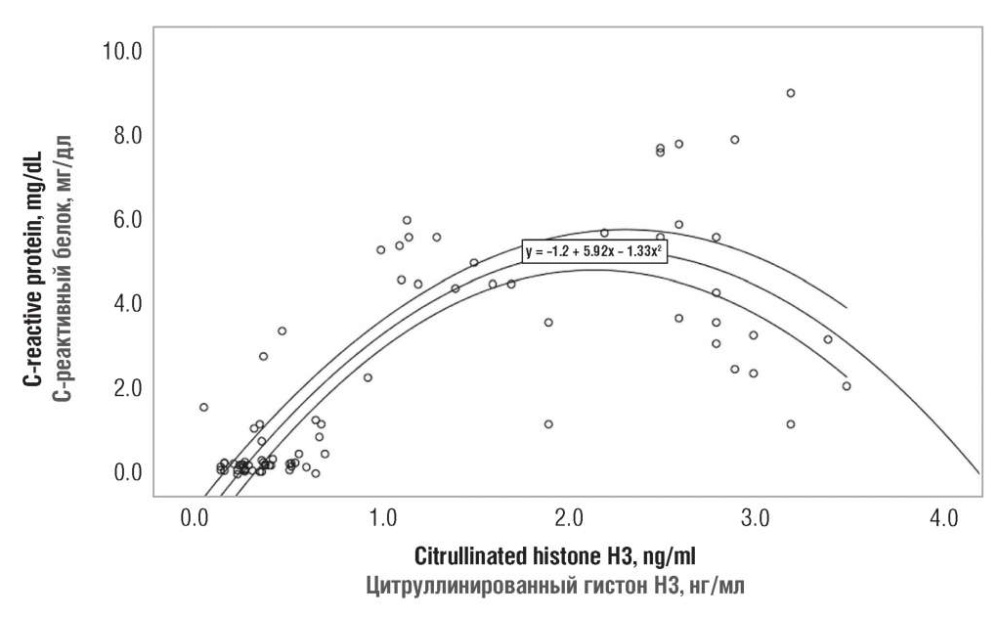
Figure 3. A relationship between serum citrullinated histone H3 and C-reactive protein level in both patient groups.
Рисунок 3. Взаимосвязь между значениями цитруллинированного гистона Н3 и С-реактивного белка в плазме крови пациенток обеих групп.
The platelet count was observed to evenly increase along with citH3 concentration. At the minimum citH3 magnitude, the platelet count did not exceed 250×109/L, but upon elevation of citH3 concentration up to 4 ng/ml, it linearly increases from 250×109/L up to 350×109/L (Fig. 4).
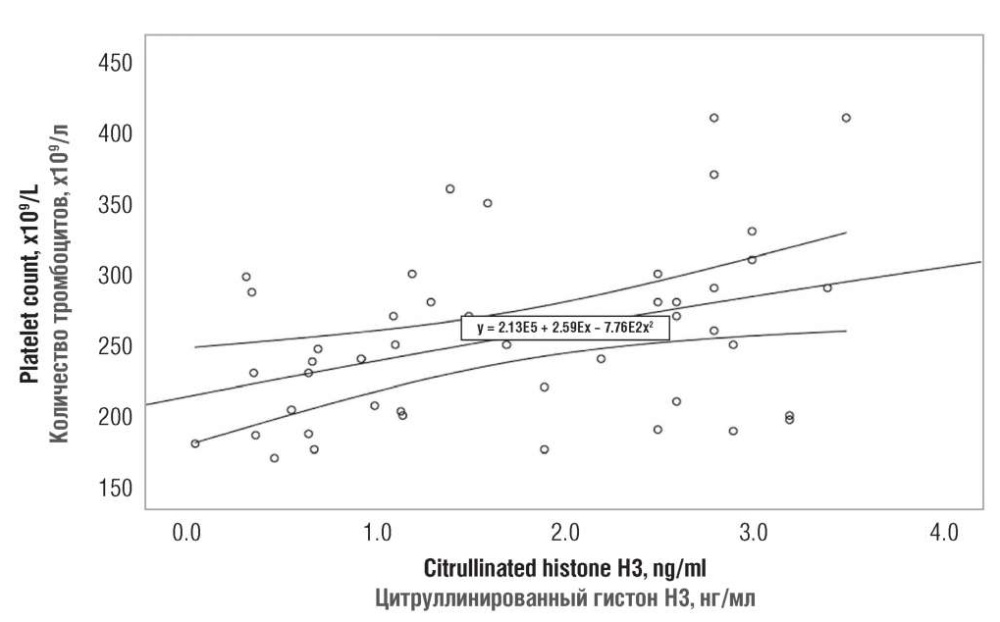
Figure 4. A relationship between serum citrullinated histone H3 and platelet count in both patient groups.
Рисунок 4. Взаимосвязь между значениями цитруллинированного гистона Н3 и числом тромбоцитов у обследованных пациенток обеих групп.
While assessing the data obtained, platelet count in the patient groups was compared by using the Mann– Whitney test and the confidence interval. As a result, a high-level significance inter-group differences were found (p = 0.0001). The confidence interval analysis shows that the platelet count in control group ranges from 178.14 to 202.35×109/L, whereas in main group – from 236.68 to 273.77×109/L (Fig. 5).
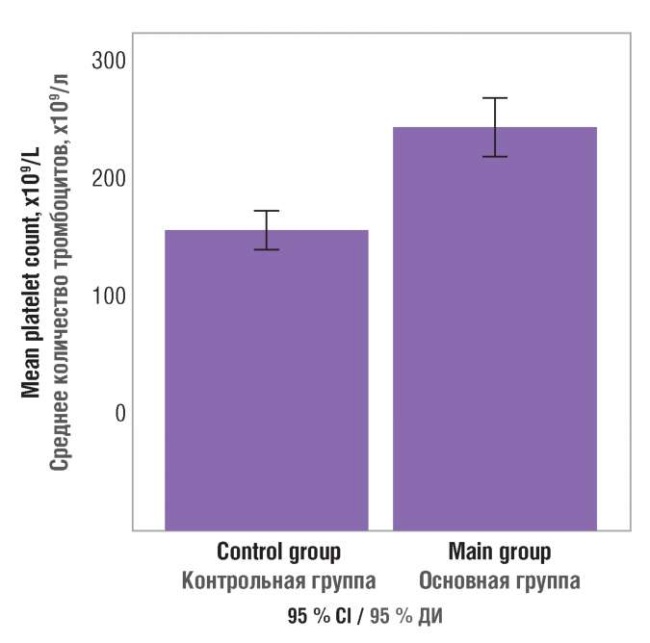
Figure 5. Blood platelet count in patients examined.
Рисунок 5. Количество тромбоцитов в крови обследованных пациенток.
Discussion / Обсуждение
Recent results from numerous studies allow us to consider neutrophils potentially as the key players in neoplastic events in addition to their role in host defense against infection. However, some evidence strongly suggests that neutrophils may play a role in tumor progression and cancer-associated thrombosis catastrophically affecting host. Unfortunately, no effective neutrophil-targeting therapy has yet been developed in the context of tumor progression and thrombotic risks. However, as our understanding of the pro-oncogenic and prothrombogenic mechanisms of neutrophils becomes deeper, there is an opportunity to develop new targets for anticancer therapy and improve patient outcomes.
Studies have shown that NETs promote tumor proliferation [30], metastasis [31], and thrombosis [32]. Previous studies reported detection of plasma NETs in various diseases such as autoimmune smallvessel vasculitis, colorectal cancer, and hepatocellular cancer [28][29][33]. In this study, for the first time a contribution of NETosis in tumor process was evaluated in uterine body, cervical (adenocarcinoma), ovarian, and breast cancer patients. The citH3 (a NETosis marker) concentration in patients with uterine body, cervical (adenocarcinoma), and ovarian tumors was higher than in patients with benign diseases, suggesting that neutrophils and NETs they released play an essential role in developing tumors of this anatomical localization.
Leukocytosis is typical for cancer patients. In the tumor process, leukocytosis mainly being accounted for by mature neutrophils. Granulocyte colony-stimulating factor (GCSF) produced by tumor tissue increases the number of neutrophils and enhances related activation. An increased neutrophil count in cancer patients is combined with an unfavorable prognosis for the disease course. Experimentally, administration of GCSF to healthy mice led to the H3 histone hypercitrulinization in neutrophils and increased NETosis. GCSF administered to mice with breast tumors increased NETs concentration, shortened bleeding time and kidney and lung microthrombosis. Neutrophil trap fragments trigger platelet activation and aggregation, erythrocyte thrombogenesis, and released the von Willebrand factor [34][35]. Our study showed that the citH3 concentration significantly correlates with neutrophil count and neutrophil/lymphocyte ratio, thereby confirming a role for neutrophils as the primary NETs sources as well as a role of NETosis in tumor growth.
A systemic inflammation is characterized by increased concentration of circulating acute-phase proteins, such as C-reactive protein. Before surgery, this parameter serves as a stage-independent prognostic factor in many types of cancer [36]. Cells involved in the systemic inflammatory response, such as neutrophils, lymphocytes, monocytes, and platelets, are of prognostic value in patients with malignant neoplasms [37]. Preoperative changes in circulating leukocytes particularly neutrophil/lymphocyte ratio have been used to predict overall and tumor-associated survival in multiple malignant tumors [38]. In addition, we uncovered that along with association between tumor process and elevated NETosis marker, there was also an increase in serum citH3 level that significantly correlated with elevated CRP concentration.
The relationship between cancer and thrombosis has been known since the time of Armand Trousseau, who described the relationship between idiopathic venous thromboembolism and latent tumor growth. Thrombosis is one of the crucial complications of the oncological process holding the second place among all causes of death in oncological patients. Some studies have shown that NETs act as a building material and, by stimulating platelet aggregation, they are involved in thrombogenesis in cancer [35]. In models of breast cancer, an increased neutrophil count was noted upon tumor growth. At the later stages of the disease, the first thromboses manifest when the serum level of free DNA (as a NETs constituent) and citH3 might be detected.
Thrombocytosis traditionally accompanies the paraneoplastic process. Many studies have shown that thrombocytosis predicts distant metastases, particularly in colorectal cancer [39]. Some types of tumor cells, e. g., ovarian tumors, can produce thrombopoietin, a cytokine that stimulates the differentiation and proliferation of megakaryocytes resulting in platelet production. Our study showed an increase in platelet levels in the patient cohort with ovarian, uterine body, cervical and breast cancer compared with control group. In addition, a strong relationship between increased platelet count and citH3 level in cancer patients was uncovered, evidencing about parallel ongoing inflammation and thrombogenesis events. A significant correlation was also found between increased citH3 concentration and D-dimer as one of the primary thrombogenesis markers suggesting about developed prothrombotic state.
Taking into account all the aforementioned, NETs can be considered as one of the prothrombogenic factors in cancer patients and a potential target for developing new options for thromboprophylaxis and antithrombotic therapy. In addition, assessing NETosis markers may be used as a screening strategy to evaluate onset of disordered hemostasis system in case when major routine assays provide unaltered data and be a marker of unfavorable prognosis for disease development.
Conclusion / Заключение
In this study an assessment was made of the severity of NETosis processes in patients with tumors of the body and cervix (adenocarcinoma) of the uterus, ovaries, and mammary glands. As a result, it was shown that in cancer patients, citH3 was significantly increased compared to the control group. The concentration of citH3 in patients with the body and cervix tumors ranges from 2.271 to 2.992 ng/ml, in patients with ovarian tumors – from 1.357 to 2.123 ng/ml, and in patients with breast tumors – from 0.331 to 0.859 ng/ml. The present study did not establish significant differences in citH3 level of patients with breast tumors compared with the control group. With an increase in citH3 concentration, such parameters as CRP, D-dimer, the concentration of neutrophils, platelets, and neutrophil/lymphocyte ratio significantly increase. There were significant differences in platelet count in the experimental group compared with the control group (236.68–273.77×109/L vs. 178.14–202.35×109/L).
The present study demonstrates only a part of the overall picture of parameters characterizing the relationship between the processes of thrombosis and tumor growth, characterized by the complexity and multidimensionality of relationships. The discovery of these relationships can lead to significant progress in their pathogenesis and diagnosis.
References
1. van Kessel K.P., Bestebroer J., van Strijp J.A. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front Immunol. 2014;5:467. https://doi.org/10.3389/fimmu.2014.00467.
2. Lazzaretto B., Fadeel B. Intra- and extracellular degradation of neutrophil extracellular traps by macrophages and dendritic cells. J Immunol. 2019;203(8):2276–90. https://doi.org/10.4049/jimmunol.1800159.
3. Nirmala J.G., Lopus M. Cell death mechanisms in eukaryotes. Cell Biol Toxicol. 2020;36(2):145–64. https://doi.org/10.1007/s10565-019-09496-2.
4. Vorobjeva N., Chernyak B. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry (Moscow). 2020;85(10):1178–90. https://doi.org/10.1134/S0006297920100065.
5. Libby P., Pasterkamp G., Crea F., Jang I.-K. Reassessing the mechanisms of acute coronary syndromes: the “vulnerable plaque” and superficial erosion. Circ Res. 2019;124(1):150–60. https://doi.org/10.1161/CIRCRESAHA.118.311098.
6. Heinecke J.W., Li W., Francis G.A., Goldstein J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91(6):2866–72. https://doi.org/10.1172/JCI116531.
7. Weinrauch Y., Drujan D., Shapiro S.D. et al. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417(6884):91–4. https://doi.org/10.1038/417091a.
8. Pertiwi K.R., de Boer O.J., Mackaaij C. et al. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J Pathol. 2019;247(4):505–12. https://doi.org/10.1002/path.5212.0.1002/path.5212.
9. Bryk A.H., Prior S.M., Plens K. et al. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: associations with a prothrombotic state and hypofibrinolysis. Cardiovasc Diabetol. 2019;18(1):1–12. https://doi.org/10.1186/s12933-019-0850-0.
10. Middleton E.A., He X.Y., Denorme F et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–79. https://doi.org/10.1182/blood.2020007008.
11. Yang D., Liu J. Neutrophil extracellular traps: A new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Research. 2021;40(1):233. https://doi.org/10.1186/s13046-021-02013-6
12. Doring Y., Soehnlein O., Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43. https://doi.org/10.1161/CIRCRESAHA.116.309692.
13. Erpenbeck L., Chowdhury C.s, Zsengellér Z.K. et al. PAD4 deficiency decreases inflammation and susceptibility to pregnancy loss in a mouse model. Biol Reprod. 2016;95(6):132. https://doi.org/10.1095/biolreprod.116.140293.
14. Streiff M.B., Abutalib S.A., Farge D. et al. Update on guidelines for the management of cancer-associated thrombosis. Oncologist. 2021;26(1):e24–e40. https://doi.org/10.1002/onco.13596.
15. Navi B.B., Reiner A.S., Kamel H. et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70(8):926–38. https://doi.org/10.1016/j.jacc.2017.06.047.
16. Lyman G.H. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011;117(7):1334–49. https://doi.org/10.1002/cncr.25714.
17. Zhou Y., Tao W., Shen F. et al. The emerging role of neutrophil extracellular traps in arterial, venous and cancer-associated thrombosis. Front Cardiovasc Med. December 2021. https://doi.org/10.3389/fcvm.2021.786387.
18. Efrimescu C.I., Buggy P.M., Buggy D.J. Neutrophil extracellular trapping role in cancer, mtastases, and cancer-related thrombosis: a narrative review of the current evidence base. Curr Oncol Rep. 2021;23(10):118. https://doi.org/10.1007/s11912-021-01103-0.
19. Chen Q., Zhang L., Li X., Zhuo W. Neutrophil extracellular traps in tumor metastasis: pathological functions and clinical applications. Cancers (Basel). 2021;13(11):2832. https://doi.org/10.3390/cancers13112832.
20. Hong D., Fritz A.J., Zaidi S.K. et al. Epithelial-to-mesenchymal transition and cancer stem cells contribute to breast cancer heterogeneity. J Cell Physiol. 2018;233(12):9136–44. https://doi.org/10.1002/jcp.26847.
21. Pieterse E., Rother N., Garsen M. et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2017;37(7):1371–9. https://doi.org/10.1161/ATVBAHA.117.309002.
22. Hedrick C.C., Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173–87. https://doi.org/10.1038/s41577-021-00571-6.
23. Martins-Cardoso K., Almeida V.H., Bagri K.M. et al. Neutrophil extracellular traps (NETs) promote pro-metastatic phenotype in human breast cancer cells through epithelial–mesenchymal transition. Cancers (Basel). 2020;12(6):1542. https://doi.org/10.3390/cancers12061542.
24. Snoderly H.T., Boone B.A., Bennewitz M.F. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019;21(1):145. https://doi.org/ 10.1186/s13058-019-1237-6.
25. Faget J., Groeneveld S., Boivin G. et al. Neutrophils and snail orchestrate the establishment of a pro-tumor microenvironment in lung cancer. Cell Rep. 2017;21(11):3190–204. https://doi.org/10.1016/j.celrep.2017.11.052.
26. Berger-Achituv S., Brinkmann V., Abu-Abed U. et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. https://doi.org/10.3389/fimmu.2013.00048.
27. Arelaki S., Arampatzioglou A., Kambas K. et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PloS One. 2016;11(5):e0154484. https://doi.org/10.1371/journal.pone.0154484.
28. Tohme S., Yazdani H.O., Al-Khafaji A.B. et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367–80. https://doi.org/10.1158/0008-5472.CAN-15-1591.
29. van der Windt D.J., Sud V., Zhang H. et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–60. https://doi.org/10.1002/hep.29914.
30. Nie M., Yang L., Bi X. et al. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res. 2019;25(6):1867–79. https://doi.org/10.1158/1078-0432.CCR-18-1226.
31. Lee W., Ko S.Y., Mohamed M.S. et al. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94. https://doi.org/10.1084/jem.20181170.
32. Wolach O., Sellar R.S., Martinod K. et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10(436):eaan8292. https://doi.org/10.1126/scitranslmed.aan8292.
33. Kessenbrock K., Krumbholz M., Schonermarck U. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–5. https://doi.org/10.1038/nm.1959.
34. Demers M., Krause D.S., Schatzberg D. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancerassociated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076–81. https://doi.org/10.1073/pnas.1200419109.
35. Demers M., Wagner D.D. Neutrophil extracellular traps: A new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology. 2013;2(2):e22946. https://doi.org/10.4161/onci.22946.
36. McMillan D., Canna K., McArdle C. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90(2):215–19. https://doi.org/10.1002/bjs.4038.
37. Hauser C.A,. Stockler M.R., Tattersall M.H. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999–1011. https://doi.org/10.1007/s00520-006-0079-9.
38. Walsh S., Cook E., Goulder F. et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–4. https://doi.org/10.1002/jso.20329.
39. Cravioto-Villanueva A., Luna-Perez P., Gutierrez-de la Barrera M. et al. Thrombocytosis as a predictor of distant recurrence in patients with rectal cancer. Arch Med Res. 2012;43(4):305–11. https://doi.org/10.1016/j.arcmed.2012.06.008.
About the Authors
E. V. SlukhanchukRussian Federation
Ekaterina V. Slukhanchuk – MD, PhD, Associate Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
V. O. Bitsadze
Russian Federation
Victoria O. Bitsadze – MD, Dr Sci Med, Professor of RAS, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 6506003478. Researcher ID: F-8409-2017
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
A. G. Solopova
Russian Federation
Antonina G. Solopova – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children's Health
Scopus Author ID: 6505479504. Researcher ID: Q-1385-2015
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
J. Kh. Khizroeva
Russian Federation
Jamilya Kh. Khizroeva – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 57194547147. Researcher ID: F-8384-2017
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
J.-Ch. Gris
Russian Federation
Jean-Christophe Gris – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health; Professor of Haematology, Head of the Laboratory of Haematology, Faculty of Biological and Pharmaceutical Sciences; Foreign Member of RAS
Scopus Author ID: 7005114260. Researcher ID: AAA-2923-2019
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
163 Rue Auguste Broussonnet, Montpellier 34090, France
I. Elalamy
Russian Federation
Ismail Elalamy – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 7003652413. Researcher ID: AAC-9695-2019
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
12 Rue de l’École de Médecine, Paris 75006, France
4 Rue de la Chine, Paris 75020, France
A. D. Makatsariya
Russian Federation
Alexander D. Makatsariya – MD, Dr Sci Med, Academician of RAS, Professor, Head of the Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
Scopus Author ID: 57222220144. Researcher ID: M-5660-2016
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991, Russia
Review
For citations:
Slukhanchuk E.V., Bitsadze V.O., Solopova A.G., Khizroeva J.Kh., Gris J., Elalamy I., Makatsariya A.D. Neutrophil extracellular traps as markers of thromboinflammation in the pathogenesis of female genital tract and breast malignant neoplasms. Obstetrics, Gynecology and Reproduction. 2022;16(4):426-437. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.335

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































