Scroll to:
Preeclampsia and venous thromboembolism
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.315
Abstract
Preeclampsia (PE) is a multisystemic disease that has been recorded as a complication in up to 15 % of pregnancies being lead cause of maternal mortality worldwide. Despite that PE pathophysiology has not been fully elucidated, it is currently believed that the endothelial dysfunction and pro-inflammatory status play a key role in its development, which account for impaired implantation processes as well as trophoblast invasion during placentation. Altogether, it results in developing generally accepted clinical symptoms “triad”: arterial hypertension, proteinuria, and edema. PE is also characterized by clotting disorders that cause an increased risk of maternal venous thromboembolism. It should be remembered that the related risk may be markedly elevated in the postpartum period. The mechanisms underlying the development of thrombosis high risk remain to be fully investigated, albeit upregulated expression of procoagulant factors, endothelial dysfunction, compromised endogenous anticoagulant activity, and increased platelet activity result in prothrombotic predisposition.
For citations:
Grigoreva K.N., Bitsadze V.O., Khizroeva J.Kh., Slukhanchuk E.V., Tretyakova M.V., Makatsariya N.A., Gris J., Di Renzo G.C., Tsibizova V.I., Blinov D.V., Makatsariya A.D. Preeclampsia and venous thromboembolism. Obstetrics, Gynecology and Reproduction. 2022;16(3):306-316. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.315
INTRODUCTION / ВВЕДЕНИЕ
Preeclampsia (PE) is a condition that develops in a large number of pregnant women and results in severe consequences. PE-related complications vary, which may include intrauterine fetal growth retardation, fetal death (1–2 % of cases), premature birth, impaired liver and kidney functions, thrombosis, coagulopathy, eclampsia, etc. [1]. Around 70,000 women die annually worldwide due to PE complications, wherein venous thromboembolism (VTE) is a lead risk factor for maternal mortality [2].
Normally, pregnancy is characterized by the development of a hypercoagulant state, however, patients with PE experience significant changes in the proand anticoagulant pathways, which leads to an "abnormal" procoagulant state. Such blood clotting disorders result in increased VTE risk, especially in the postpartum period [3]. Despite this, therapeutic strategies for PE treatment and prevention currently remain poorly studied, whereas a childbirth is the only effective treatment method. Eliminating such knowledge gaps may lead to lowered morbidity and mortality of both mothers and children affected by PE.
Here, we review potential pathogenetic mechanisms related to PE and VTE occurring during PE as well as discuss current methods for prevention and treatment of such conditions.
PATHOGENETIC ASPECTS OF PREECLAMPSIA DEVELOPMENT / ПАТОГЕНЕТИЧЕСКИЕ АСПЕКТЫ РАЗВИТИЯ ПРЕЭКЛАМПСИИ
Preeclampsia (PE) is a multisystemic inflammatory disease that accounts for up to 15 % of maternal deaths worldwide [2][4]. Both maternal and placental factors influence PE development. The placenta plays an essential role in the pathophysiology of emerging PE, especially early PE that was corroborated by experimental data showing that placental tissue rather than fetus is necessary for the disease development [5]. Investigating human placenta at various stages of gestation in women with normal pregnancies or PE provided insights into the normal placental morphology and pathological changes in the uteroplacental circulation likely to be related to PE. It is clear that defects in spiral artery remodeling and trophoblast invasion, two interrelated but separate processes, are characteristic of hypertensive disorders of pregnancy and fetal growth retardation [6]. Anomalies in the development of placental vessels in early pregnancy can lead to relative placental hypoperfusion/hypoxia/ ischemia, which then contributes to the release of antiangiogenic factors into maternal bloodstream resulting in development of a pro-inflammatory state and endothelial dysfunction [7][8].
Numerous studies demonstrated a role for the endothelial dysfunction in PE [9] resulting in endothelium dysfunction leads to the formation of vasospasm, increased vascular permeability, and activation of blood coagulation system, together underlying the development of clinical symptoms [10–12]. For example, hypertension results from impaired endothelial control of vascular tone, but proteinuria and edema develop due to increased vascular permeability, whereas coagulopathy is caused by abnormal endothelial procoagulant expression. Headache, seizures, epigastric pain, and fetal growth retardation are consequences of endothelial dysfunction in the target organ vasculature including brain, liver, kidneys, and placenta.
Usually, intact endothelium bears a surface negative charge exerting diverse anticoagulant properties that inhibit intravascular thrombogenesis and platelet activation. The negatively charged glycosaminoglycan layer forming the glycocalyx that lines up the luminal endothelial surface inhibits thrombin formation by interacting with circulating endogenous anticoagulants (such as antithrombin) and also inhibits leukocyte and platelet adhesion. Nitric oxide and prostacyclins also limit coagulation activation by inhibiting platelet activation and counteracting vasoconstriction. Moreover, the physiological endothelial expression of anticoagulant proteins such as thrombomodulin, endothelial protein C receptor, and tissue plasminogen activator are crucial to the activation of protein C and the fibrinolytic system [13][14].
Endothelial glycocalyx degradation was described in early-onset PE that appears to be associated with reduced microvascular perfusion. Decreased thrombomodulin endothelial expression (due to the cleavage from the cell surface by leukocyte proteases and metalloproteases) is a well-known marker of endothelial dysfunction in response to acute inflammation [15][16]. Importantly, a decreased physiological placental thrombomodulin expression in PE correlates with level of the anti-angiogenic factor – sFlt-1 (soluble fms-like tyrosinekinase 1). The developing placenta produces various pro-angiogenic factors such as VEGF (vascular endothelial growth factor), PlGF (placental growth factor) and anti-angiogenic factor sFlt-1, and the balance between them is essential for normal placental development. In PE, the imbalance is characterized by excessive level of anti-angiogenic factors [17], including sFlt-1 and soluble endoglin (sEng), combined with decreased physiological levels of pro-angiogenic proteins VEGF and PlGF. These markers are used clinically during first-trimester screening as diagnostic/prognostic biomarkers [18][19]. The International Federation of Gynecology and Obstetrics (FIGO) recommends to using such biomarkers in the "screening and prevention" strategy for first trimester-PE development.
Soluble fms-like tyrosine kinase 1 is a natural circulating VEGF antagonist, wherein VEGF is an endothelial-specific mitogen playing a crucial role in stimulating angiogenesis [20]. Its activity is mainly mediated by interaction with high-affinity receptor tyrosine kinases. Soluble fmslike tyrosine kinase 1 counteracts the pro-angiogenic activity of serum VEGFs by binds them and preventing from interaction with cognate endogenous receptors. Increased placental sFlt-1 expression and secretion appear to play a central role in the PE pathogenesis [20–23]. In PE, the etiology of endothelial dysfunction is multifactorial, but placental-derived factors seem to be vital in inducing endothelial cell damage, including sFlt-1. In particular, the study with pregnant rats injected with sFlt-1 was noted to have albuminuria, hypertension as well as pathological glomerular changes [24].
Preeclampsia-induced endothelial dysfunction leads to elevated release of endothelial extracellular vesicles (a hallmark of endothelial cell damage) shown to mediate pro-inflammatory and prothrombotic effects. A population of extracellular vesicles activates pathological signaling pathways on platelets, neutrophils, and other leukocytes [25]. In particular, in PE they can induce NETosis – a type of regulated neutrophil cell death characterized by the release of neutrophil extracellular traps (NETs) in response to inflammatory signals, which appears to be an essential mechanism promoting activation of the coagulation and inflammatory pathways [26]. Neutrophil extracellular traps are the networks composed of DNA, histones, and proteins produced by activated neutrophils. Their crucial role in the initiation of immune neutrophil response, the pathogenesis of autoimmune conditions such as systemic lupus erythematosus, rheumatoid arthritis, psoriasis as well as other non-infectious processes, such as coagulation disorders, thrombosis, diabetes, atherosclerosis, vasculitis, and oncological diseases has been confirmed. NETs promote clotting activation by activating platelets and direct activation of circulating blood coagulation factors [27][28].
Defects in trophoblast invasion may also be associated with compromised immunological tolerance to a "semiallotransplant" fetus. Immune mechanisms at the maternalplacental interface may be multifactorial, including deficiency of natural killer cells early in placentation and abnormal recognition of paternal HLA-C (human leukocyte antigens) by maternal killer Ig-like receptors. In addition, PE is a pro-inflammatory condition in which interleukin-10 (IL-10) and pro-inflammatory cytokine level, including IL-12 and IL-18 becomes dysregulated along with increased complement system level [29–31].
In addition, a correlation between obesity and PE was also observed. A prospective study demonstrated a linear relationship between increased body mass index (BMI) and higher risk of developing PE [32]. In this cohort, the odds of developing PE in women with BMI 25 to 30 kg/m2 vs. ≥ 40 kg/m2 increased from 1.65 up to 6.04. Highly likely, obesity increases the predisposition to PE by inducing chronic inflammation and endothelial dysfunction, which may induce PE microangiopathic signs in synergy with placental angiogenic factors [33]. Together, all these processes lead to systemic vascular dysfunction and maternal disorders (Fig. 1).
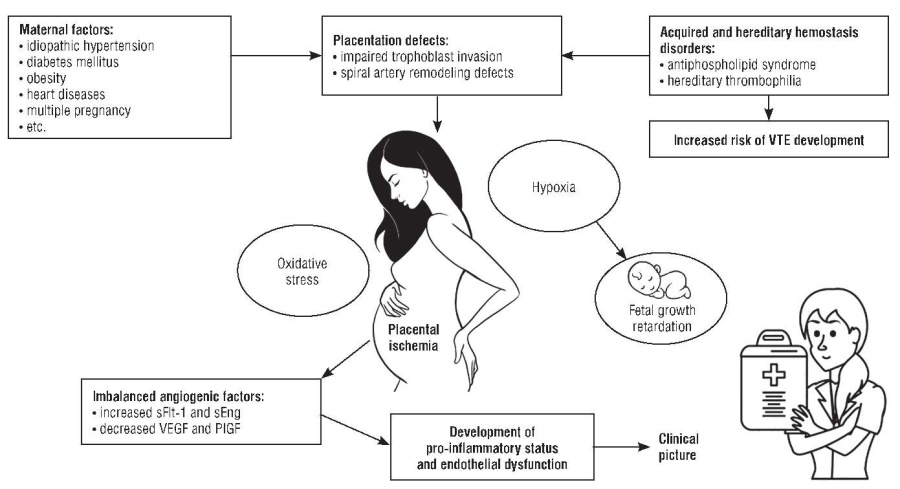
Figure 1. Pathogenetic aspects of preeclampsia (drawn by authors based on data from [34]).
Note: VTE – venous thromboembolism; sFlt-1 – soluble fms-like tyrosine kinase 1; sEng – soluble endoglin; VEGF – vascular endothelial growth factor; PlGF – placental growth factor.
Рисунок 1. Патогенетические аспекты преэклампсии (рисунок авторов по данным из статьи [34]).
Примечание: VTE – венозная тромбоэмболия; sFlt-1 – растворимая fms-подобная тирозинкиназа 1; sEng – растворимый эндоглин; VEGF – фактор роста эндотелия сосудов; PlGF – плацентарный фактор роста.
ABNORMAL SPIRAL ARTERY REMODELING / АНОМАЛЬНОЕ РЕМОДЕЛИРОВАНИЕ СПИРАЛЬНЫХ АРТЕРИЙ
In normal pregnancy, cytotrophoblast cells migrate through the decidua and part of the myometrium, infiltrating both the endothelium and the muscular propria of the maternal spiral arteries, the terminal branches of the uterine artery supplying blood to the developing placenta. As a result, these vessels transform from small muscular arterioles to low-resistance "large vessels," which significantly facilitate blood flow to the placenta compared to other uterine areas. For comparison, in PE, cytotrophoblast cells infiltrate the decidual part of the spiral arteries but do not penetrate the myometrial segment; as a result, the spiral arteries cannot transform into large tortuous vascular channels. Some scientists believe that the defective trophoblast differentiation is one of the potential mechanisms responsible for incorrect cytotrophoblast invasion into the spiral arteries [35].
VENOUS THROMBOEMBOLISM IN PREGNANCY / ВЕНОЗНАЯ ТРОМБОЭМБОЛИЯ У БЕРЕМЕННЫХ
Usually, pregnancy is characterized by developing hypercoagulable state, an increased activity of the procoagulant factor, as well as the suppression of endogenous anticoagulant and fibrinolytic pathways. The hypercoagulable state is thought to develop to limit the risk of major bleeding associated with childbirth [36]. Pregnancy-associated hypercoagulability may reduce the risk of major perinatal bleeding, but a shift towards a procoagulant phenotype also increases a chance of VTE development.
The risk of VTE during pregnancy increases by about six-fold compared with age-matched non-pregnant women [37]. Venous thromboembolism complicates about 1–2 out of 1000 pregnancies. Thus, in the United States as well as the UK and Ireland, VTE is the sixth and first lead cause of maternal death [38][39]. The risk is higher in women with previous VTE episodes, verified hereditary or acquired thrombophilia, and women with positive family history. The magnitude of the risk depends on whether the pre-pregnancy VTE was unprovoked (3.6 %), provoked (1.1 %), or associated with the intake of exogenous hormones (6.4 %) [40]. Women with hereditary thrombophilia have a 15 times higher risk of VTE development than in remaining pregnant women (95 % confidence interval (CI) = 10.8–22.0), with the absolute risk of deep vein thrombosis (DVT) and pulmonary embolism being 146 per 10,000 and 43 per 10,000, respectively [41]. Around 80 % of pregnancyassociated VTEs are manifested as DVT symptoms, whereas the remaining 20 % are presented as pulmonary embolism or DVT combined with pulmonary embolism.
Taking into account a potential impact of thrombophilia on duration of treatment, prenatal care, and associated complications, guidelines from the American College of Gynecologists (ACOG), the Society of Obstetricians and Gynecologists of Canada (SOGC), and the Royal College of Obstetricians and Gynecologists (RCOG) support relevance to test all pregnant women with a history of VTE for antiphospholipid syndrome and hereditary thrombophilia, including factor V Leiden (FVL) and prothrombin gene G20210A (PT G20210A), as well as deficiency of antithrombin III, protein C and protein S [42][43].
It should be remembered that VTE risk increases with age, in the presence of obesity and/or comorbidities (e.g., hypertensive disorders, systemic lupus erythematosus), and with delivery by cesarean section [44][45]. Assisted reproductive technologies, including ovarian stimulation, also increase VTE risk by 2–3-fold compared with the general population of pregnant women. This is due to supraphysiological estradiol levels, which lead to hemoconcentration as well as activation of the coagulation and fibrinolytic systems [46].
RISK OF VENOUS THROMBOEMBOLISM IN PREGNANT WOMEN WITH PREECLAMPSIA / РИСК РАЗВИТИЯ ВЕНОЗНОЙ ТРОМБОЭМБОЛИИ У БЕРЕМЕННЫХ С ПРЕЭКЛАМПСИЕЙ
Preeclampsia complicates many pregnancies being the lead cause of maternal and infant mortality, wherein therapeutic strategies remain poorly understood. The increased baseline VTE risk associated with pregnancy is increased due to additional factors such as PE [47–49]. It is important to note that women diagnosed with PE have a risk of developing VTE that varies depending on the "stage" of pregnancy (the highest risk phase is the postpartum period, about 50 % of pregnancy-related VTE occurs within the first six weeks after delivery) and PE severity (most likely due to altered procoagulant and anticoagulant pathways) [50][51]. This observation has been most clearly illustrated by several large studies showing that VTE risk associated with PE persists throughout the postpartum period and occurs three or four times more often [28][52]. This highlights the importance of assessing the VTE risk to counter such factors in early pregnancy, postpartum period, and in case of changing risk factors [44].
In addition to increased VTE risk, PE is also associated with higher risk of future cardiovascular disease, with which it shares several etiological/predisposing factors such as obesity, chronic hypertension, chronic kidney disease, etc. [53][54]. It was previously stated that PE is a disease resulting from defective arterial invasion by placental tissues. The increased risk of arterial and venous thrombosis in PE probably arises due to interplay between maternal risk factors (such as pre-existing cardiovascular disease and obesity), pregnancy-specific risk factors (such as physiological hypercoagulability), systemic endothelial dysfunction as well as PE-specific inflammatory response [55].
PREDICTIONS FOR POST-PREECLAMPSIA WOMEN / ПРОГНОЗЫ ДЛЯ ЖЕНЩИН, ПЕРЕНЕСШИХ ПРЕЭКЛАМПСИЮ
A 2015 study assessing the data from more than 75,000 women with PE in previous pregnancy found that 16 % and 20 % subjects also developed preeclampsia during their subsequent pregnancy as well as hypertension, respectively [56]. However, the risk of preeclampsia recurrence in subsequent pregnancies depends on the severity and timing of the initial episode [57]. Thus, the chances of PE developing during the second gestation were markedly lower (from 5 to 7 %) in women who had PE in first pregnancy lacking severe clinical signs and severe complications, and comprised less than 1 % in women with normotensive first pregnancy [58][59]. In contrast, women with the early-onset severe PE in previous pregnancy were at highest risk of recurrence ranging from 25 to 65 % [60][61].
In addition to high risk of developing VTE and new PE cases in subsequent pregnancies, patients with PE also have increased risk of cardiovascular disease in the future [54][62]. In 2019, a large population-based cohort study was conducted in the UK that demonstrated an almost two-fold increase in the risk of cardiovascular events (including stroke, myocardial infarction, and peripheral disease arteries) after being diagnosed with PE or other hypertensive disorder during pregnancy [63]. It was also suggested that PE increases the risk of cardiovascular diseases not only in puerperant women but also in their children, so higher magnitude indicators for chronic hypertension, dyslipidemia, and obesity were recorded among an adult population exposed to this disorder in utero. This observation reveals some genetic predisposition in PE etiology [64]. Preeclampsia pathogenesis is characterized by long-term chronic intravascular coagulation, which leads to adverse consequences, including the shortened maternal life expectancy.
PREVENTION OF VENOUS THROMBOEMBOLISM IN PREECLAMPSIA / ПРОФИЛАКТИКА ВОЗНИКНОВЕНИЯ ВЕНОЗНОГО ТРОМБОЭМБОЛИЗМА ПРИ ПРЕЭКЛАМПСИИ
The current guidelines suggest considering an opportunity for conducting a thromboprophylaxis, especially in the postpartum period, necessarily taking in consideration additional risks such as early-onset PE and intrauterine growth retardation if the VTE overall risk is more than 1–3 % [65]. Currently, pharmacological thromboprophylaxis, when necessary, is carried out by introducing low molecular weight heparin (LMWH) or unfractionated heparin (UFH) [66] displaying most favorable safety profile compared with warfarin, which crosses the placenta and is associated with increased rate of miscarriage, congenital anomalies, intrauterine bleeding, and long-term neurological consequences [67] or direct oral anticoagulants also penetrating the placenta, which clinical impact on fetal outcomes, however, has not been established [68][69].
Currently, no studies with pregnant women directly comparing side-by-side LMWH vs. UFH prophylaxis have been carried out; in non-pregnant women, LMWH was as safe and effective as UFH [70]. LMWH exerts a more predictable response and lower incidence of osteoporosisas well as heparin-induced thrombocytopenia (HIT), although UFH may be preferred in patients with renal dysfunction (glomerular filtration rate < 30 ml/min) or in those patients who may require rapid drug discontinuation (e.g., before surgery) [71]. The selection of patients who need anticoagulant therapy is determined based on assessing VTE risk, which should be carried out before and after delivery. However, no evidence for supporting an optimal risk threshold at which thromboprophylaxis should be initiated or for optimal length of anticoagulant therapy are proposed, despite frequent emergence of occurring postpartum VTE. In a broad sense, the benefits of pharmacological VTE prophylaxis should outweigh the risk of bleeding and other complications [72]. Assessing VTE risk for each woman is critical, especially in PE, because a confirmed competing hemorrhagic risk associated with it exists. A nationwide cohort study in the Netherlands showed that 7.4 % vs. 4.2 % of women with vs. without PE developed postpartum hemorrhage, respectively [73]. Despite the established VTE risk during pregnancy, thromboprophylaxis is not beneficial for all women. Thromboembolism prevention is associated with almost 2 % risk of maternal bleeding, heparin-associated osteoporosis, and HIT [39][74]. It is important to remember that most bleeding during labor is secondary and occurs more frequently at birth/placental separation. In this case, bleeding is controlled by myometrial contractions (with blockage of uterine blood vessels) rather than by blood clotting factors targeted by anticoagulant therapy. Alternatively, bleeding from soft tissue ruptures and intrapartum trauma is sensitive to anticoagulant therapy [75].
At present, recommendations are based on expert opinion rather than high-quality evidence [76][77], which is highly challenging for physicians, especially given competing risks and issues related to pharmacological thromboprophylaxis. However, the data published to date suggest that women with severe thrombophilia or a history of VTE undoubtedly require thromboprophylaxis. In addition, anticoagulant therapy is effective in antenatal women with unprovoked or hormone-associated VTE as well as postpartum period in women with any previous VTE, regardless of etiology [77]. The most significant reduction in VTE risk during anticoagulant prophylaxis occurs in patients with a VTE familial history and with a homozygous FV (Leiden factor) mutation or a homozygous PT G20210A (prothrombin G20210A) gene mutation. In such women, the risk of VTE developing in the prenatal and postpartum was 47 per 1000 subjects. Moreover, in patients with antithrombin III, protein C, or protein S deficiency, prophylaxis led to decline in VTE rate by 13 per 1000 people. Subjects without a "gender positive" VTE familial history or bearing heterozygous variants had it decreased by 13 and 10 subjects per 1000 people, respectively [65]. In patients with antiphospholipid syndrome and history of recurrent miscarriage, combined prophylactic lowdose acetylsalicylic acid and heparin can reduce a risk of miscarriage by up to 50 % and should be considered for postpartum use for up to 6 weeks [78].
CONCLUSION / ЗАКЛЮЧЕНИЕ
Preeclampsia is a severe disease that complicates many pregnancies and is a significant cause of maternal morbidity and mortality. The mechanisms underlying this condition are not fully investigated; however, increased production of anti-angiogenic factors, endothelial dysfunction, and increased platelet activation are well recognized hallmarks of hemostatic dysfunction. It is important to remember that during pregnancy, the risks of developing VTE increases, whereas upon PE development, they may be elevated by 3–4 times. Hence, it is also crucial to assess risks of developing VTE and prevent them timely.
References
1. English F.A., Kenny L.C., McCarthy F.P. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015;8:7–12. https://doi.org/10.2147/IBPC.S50641.
2. Egan K., Kevane B., NíAinle F. Elevated venous thromboembolism risk in preeclampsia: molecular mechanisms and clinical impact. Biochem Soc Trans. 2015;43(4):696–701. https://doi.org/10.1042/BST20140310.
3. Gris J.-C., Bouvier S., Cochery-Nouvellon É. et al. The role of haemostasis in placenta-mediated complications. Thromb Res. 2019;181 Suppl 1:S10– S14. https://doi.org/10.1016/S0049-3848(19)30359-7.
4. Ramlakhan K.P., Johnson M.R., Roos-Hesselink J.W. Pregnancy and cardiovascular disease. Nat Rev Cardiol. 2020;17(11):718–31. https://doi.org/10.1038/s41569-020-0390-z.
5. Matsuo K., Kooshesh S., Dinc M. et al. Late postpartum eclampsia: report of two cases managed by uterine curettage and review of the literature. Am J Perinatol. 2007;24(4):257–66. https://doi.org/10.1055/s-2007-976548.
6. Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939– 58. https://doi.org/10.1016/j.placenta.2005.12.006.
7. Rana S., Lemoine E., Granger J.P., Karumanchi S.A. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–112. https://doi.org/10.1161/CIRCRESAHA.118.313276.
8. Guerby P., Tasta O., Swiader A. et al. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021;40:101861. https://doi.org/10.1016/j.redox.2021.101861.
9. Sánchez-Aranguren L.C., Prada C.E., Riaño-Medina C.E., Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. 2014;5:372. https://doi.org/10.3389/fphys.2014.00372.
10. System syndromes in obstetric-gynecological clinic: а guide for physicians. [Sistemnye sindromy v akushersko-ginekologicheskoj klinike: rukovodstvo dlya vrachej. Pod red. A.D. Makatsariya]. Moscow: MIA, 2010. 897 p. (In Russ.).
11. Lamarca B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol. 2012;64(4):309–20.
12. Fakhouri F., Vercel C., Frémeaux-Bacchi V. Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol. 2012;7(12):2100–6. https://doi.org/10.2215/CJN.13121211.
13. Wang M., Hao H., Leeper N.J., Zhu L.; Early Career Committee. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol. 2018;38(6):e90–e95. https://doi.org/10.1161/ATVBAHA.118.310367.
14. Weissgerber T.L., Garcia-Valencia O., Milic N.M. et al. Early onset preeclampsia is associated with glycocalyx degradation and reduced microvascular perfusion. J Am Heart Assoc. 2019;8(4):e010647. https://doi.org/10.1161/JAHA.118.010647.
15. Martin F.A., Murphy R.P., Cummins P.M. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304(12):H1585–97. https://doi.org/10.1152/ajpheart.00096.2013.
16. Turner R.J., Bloemenkamp K.W., Bruijn J.A., Baelde H.J. Loss of thrombomodulin in placental dysfunction in preeclampsia. Arterioscler Thromb Vasc Biol. 2016;36(4):728–35. https://doi.org/10.1161/ATVBAHA.115.306780.
17. Fraser R., Whitley G.S., Johnstone A.P. et al. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol. 2012;228(3):322–32. https://doi.org/10.1002/path.4057.
18. Hayes-Ryan D., Khashan A.S., Hemming K. et al. Placental growth factor in assessment of women with suspected pre-eclampsia to reduce maternal morbidity: a stepped wedge cluster randomised control trial (PARROT Ireland). BMJ. 2021;374:n1857. https://doi.org/10.1136/bmj.N1857.
19. Poon L.C., Magee L.A., Verlohren S. et al. A literature review and best practice advice for second and third trimester risk stratification, monitoring, and management of pre-eclampsia: compiled by the pregnancy and non-communicable diseases committee of FIGO (the international federation of gynecology and obstetrics). Int J Gynaecol Obstet. 2021;154 Suppl 1:3–31. https://doi.org/10.1002/ijgo.13763.
20. Dvorak H.F. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–80. https://doi.org/10.1200/JCO.2002.10.088.
21. Levine R.J., Maynard S.E., Qian C. et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. https://doi.org/10.1056/NEJMoa031884.
22. McKeeman G.C., Ardill J.E., Caldwell C.M. et al. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191(4):1240–6.https://doi.org/10.1016/j.ajog.2004.03.004.
23. Chaiworapongsa T., Romero R., Espinoza J. et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190(6):1541–7; discussion 1547–50. https://doi.org/10.1016/j.ajog.2004.03.043.
24. Maynard S.E., Min J.Y., Merchan J. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. https://doi.org/10.1172/JCI17189.
25. Hu Y., Yan R., Zhang C. et al. HMGB1 from hypoxic trophoblasts promotes endothelial microparticle production and thrombophilia in preeclampsia. Arterioscler Thromb Vasc Biol. 2018;38(6):1381–91. https://doi.org/10.1161/ATVBAHA.118.310940.
26. Hu Y., Li H., Yan R. et al. Increased neutrophil activation and plasma DNA levels in patients with pre-eclampsia. Thromb Haemost. 2018;118(12):2064–73. https://doi.org/10.1055/s-0038-1675788.
27. Slukhanchuk E.V. NETs and oncologic process. Obstetrics, Gynecology and Reproduction. 2021;15(1):107–16. (In Russ.). https://doi.org/10.17749/2313-7347/ob.gyn.rep.2021.204.
28. Jacobsen A.F., Skjeldestad F.E., Sandset P.M. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost. 2008;6(6):905–12. https://doi.org/10.1111/j.1538-7836.2008.02961.x.
29. Peixoto A.B., Araujo Júnior E., Ribeiro J.U. et al. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J Matern Fetal Neonatal Med. 2016;29(1):75–9. https://doi.org/10.3109/14767058.2014.987117.
30. Regal J.F., Burwick R.M., Fleming S.D. The complement system and preeclampsia. Curr Hypertens Rep. 2017;19(11):87. https://doi.org/10.1007/s11906-017-0784-4.
31. Sudo M., Yoshita K., Ito Y. et al. Histopathological features of kidney and renal prognosis in patients with preeclampsia. Pregnancy Hypertens. 2021;25:75–80. https://doi.org/10.1016/j.preghy.2021.05.015.
32. Paré E., Parry S., McElrath T.F. et al. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol. 2014;124(4):763–70. https://doi.org/10.1097/AOG.0000000000000451.
33. Zera C.A., Seely E.W., Wilkins-Haug L.E. et al. The association of body mass index with serum angiogenic markers in normal and abnormal pregnancies. Am J Obstet Gynecol. 2014;211(3):247.e1–7. https://doi.org/10.1016/j.ajog.2014.03.020.
34. Raia-Barjat T., Edebiri O., Ni Ainle F. Preeclampsia and venous thromboembolism: pathophysiology and potential therapy. Front Cardiovasc Med. 2022;9:856923. https://doi.org/10.3389/fcvm.2022.856923.
35. Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–5. https://doi.org/10.1161/HYPERTENSIONAHA.107.107607.
36. Chunilal S.D., Bates S.M. Venous thromboembolism in pregnancy: diagnosis, management and prevention. Thromb Haemost. 2009;101(3):428–38.
37. Parunov L.A., Soshitova N.P., Ovanesov M.V. et al. Epidemiology of venous thromboembolism (VTE) associated with pregnancy. Birth Defects Res C Embryo Today. 2015;105(3):167–84. https://doi.org/10.1002/bdrc.21105.
38. Konstantinides S.V., Meyer G., Becattini C. et al., ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. https://doi.org/10.1093/eurheartj/ehz405.
39. Pregnancy Mortality Surveillance System. Center for Disease Control and Prevention, 2019. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortalitysurveillance-system.htm.
40. Rodger M. Pregnancy and venous thromboembolism: ’TIPPS’ for risk stratification. Hematol Am Soc Hematol Educ Program. 2014;2014(1):387–92. https://doi.org/10.1182/asheducation-2014.1.387.
41. Liu S., Rouleau J., Joseph K.S. et al. Epidemiology of pregnancyassociated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can. 2009;31(7):611–20. https://doi.org/10.1016/S1701-2163(16)34240-2.
42. ACOG Practice Bulletin No. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132(1):e1–е17. https://doi.org/10.1097/AOG.0000000000002706.
43. Bates S.M., Middeldorp S., Rodger M. et al. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):92–128. https://doi.org/10.1007/s11239-015-1309-0.
44. Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium (Greentop Guideline No 37a). London: RCOG, 2015. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg37a.pdf.
45. Linnemann B., Bauersachs R., Rott H. et al.; Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism – position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45(2):87–101. https://doi.org/10.1024/0301-1526/a000503.
46. Sennstrom M., Rova K., Hellgren M. et al. Thromboembolism and in vitro fertilization–a systematic review. Acta Obstet Gynecol Scand. 2017;96(9):1045–52. https://doi.org/10.1111/aogs.13147.
47. Sultan A.A., West J., Grainge M.J. et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ. 2016;355:i6253. https://doi.org/10.1136/bmj.i6253.
48. Sultan A.A., Tata L.J., West J. et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood. 2013;121(19):3953–61. https://doi.org/10.1182/blood-2012-11-469551.
49. Osol G., Moore L.G. Maternal uterine vascular remodeling during pregnancy. Microcirculation. 2014;21(1):38–47. https://doi.org/10.1111/micc.12080.
50. Sultan A.A., West J., Tata L.J. et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156(3):366–73. https://doi.org/10.1111/j.1365-2141.2011.08956.x.
51. Zhou Z.-H., Chen Y., Zhao B.-H. et al. Early postpartum venous thromboembolism: risk factors and predictive index. Clin Appl Thromb Hemost. 2019;25:1076029618818777. https://doi.org/10.1177/1076029618818777.
52. Sultan A.A., Grainge M.J., West J. et al. Impact of risk factors on the timing of first postpartum venous thromboembolism: a population-based cohort study from England. Blood. 2014;124(18):2872–80. https://doi.org/10.1182/blood-2014-05-572834.
53. Benschop L., Duvekot J.J., van Lennep R.J.E. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105(16):1273–8. https://doi.org/10.1136/heartjnl-2018-313453.
54. Thilaganathan B., Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 2019;73(3):522–31. https://doi.org/10.1161/HYPERTENSIONAHA.118.11191.
55. Craici I., Wagner S., Garovic V.D. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis. 2008;2(4):249–59. https://doi.org/10.1177/1753944708094227.
56. van Oostwaard M.F., Langenveld J., Schuit E. et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am J Obstet Gynecol. 2015;212(5):624.e1–17. https://doi.org/10.1016/j.ajog.2015.01.009.
57. Barton J.R., Sibai B.M. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112(2 Pt 1):359–72. https://doi.org/10.1097/AOG.0b013e3181801d56.
58. Mostello D., Kallogjeri D., Tungsiripat R., Leet T. Recurrence of preeclampsia: effects of gestational age at delivery of thefirst pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol. 2008;199(1):55.e1–7. https://doi.org/10.1016/j.ajog.2007.11.058.
59. van Rijn B.B., Hoeks L.B., Bots M.L. et al. Outcomes of subsequent pregnancy after first pregnancy with early-onset preeclampsia. Am J Obstet Gynecol. 2006;195(3):723–8. https://doi.org/10.1016/j.ajog.2006.06.044.
60. Bramham K., Briley A.L., Seed P. et al. Adverse maternal and perinatal outcomes in women with previous preeclampsia: a prospective study. Am J Obstet Gynecol. 2011;204(6):512.e1–9. https://doi.org/10.1016/j.ajog.2011.02.014.
61. McDonald S.D., Best C., Lam K. The recurrence risk of severe de novo pre-eclampsia in singleton pregnancies: a population-based cohort. BJOG. 2009;116(12):1578–84. https://doi.org/10.1111/j.1471-0528.2009.02317.x.
62. Benschop L., Duvekot J.J., Roeters van Lennep J.E. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105(16):1273–8.
63. Leon L.J., McCarthy F.P., Direk K. et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation. 2019;140(13):1050–60. https://doi.org/10.1161/HYPERTENSIONAHA.118.038080.
64. Alsnes I.V., Vatten L.J., Fraser A. et al. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (Nord-Trøndelag Health Study) in Norway. Hypertension. 2017;69(4):591–8. https://doi.org/10.1161/HYPERTENSIONAHA.116.08414.
65. Bates S.M., Greer I.A., Middeldorp S. et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S–736S. https://doi.org/10.1378/chest.11-2300.
66. Kelliher S., Maguire P.B., Szklanna P.B. et al. Pathophysiology of the venous thromboembolism risk in preeclampsia. Hamostaseologie. 2020;40(5):594–604. https://doi.org/10.1055/a-1162-3905.
67. Schaefer C., Hannemann D., Meister R. et al. Vitamin K antagonists and pregnancy outcome. A multi-centre prospective study. Thromb Haemost. 2006;95(6):949–57. https://doi.org/10.1160/TH06-02-0108.
68. Mohzari Y.A., Asdaq S.M.B., Bamogaddam R.F. et al. Postpartum prophylaxis of venous thromboembolism with anticoagulation: a case report. J Taibah Univ Med Sci. 2021;16(2):292–4. https://doi.org/10.1016/j.jtumed.2020.12.016.
69. Sessa M., Mascolo A., Callreus T. et al. Direct-acting oral anticoagulants (DOACs) in pregnancy: new insight from VigiBase®. Sci Rep. 2019;9(1):7236. https://doi.org/10.1038/s41598-019-43715-4.
70. Quinlan D.J., Mcquillan A., Eikelboom J.W. Low molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140(3):175–83. https://doi.org/10.7326/0003-4819-140-3-200402030-00008.
71. Galambosi P., Hiilesmaa V., Ulander V.M. et al. Prolonged low molecularweight heparin use during pregnancy and subsequent bone mineral density. Thromb Res. 2016;143:122–6. https://doi.org/10.1016/j.thromres.2016.05.016.
72. Bapat P., Pinto L.S., Lubetsky A. et al. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost. 2016;14(7):1436–41. https://doi.org/10.1111/jth.13353.
73. von Schmidt auf Altenstadt J.F., Hukkelhoven C.W.P.M., van Roosmalen J., Bloemenkamp K.W.M. Pre-eclampsia increases the risk of postpartum haemorrhage: a nationwide cohort study in the Netherlands. PLoS One. 2013;8(12):e81959. https://doi.org/10.1371/journal.pone.0081959.
74. Rodger M.A., Gris J.C., de Vries J.I.P. et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: a metaanalysis of individual patient data from randomised controlled trials. Lancet. 2016;388(10060):2629–41. https://doi.org/10.1016/S0140-6736(16)31139-4.
75. Leduc D., Senikas V., Lalonde A.B., Clinical Practice Obstetrics Committee. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31:980–93. https://doi.org/10.1016/S1701-2163(16)34329-8.
76. Chan W.-S., Rey E., Kent N.E. et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527–53. https://doi.org/10.1016/s1701-2163(15)30569-7.
77. Bates S.M., Rajasekhar A., Middeldorp S. et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317–59. https://doi.org/10.1182/bloodadvances.2018024802.
78. Branch D.W., Holmgren C., Goldberg J.D. Committee on Practice Bulletins – Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: antiphospholipid antibody syndrome. Obstet Gynaecol. 2012;120(6):1514–21. https://doi.org/10.1097/01.AOG.0000423816.39542.0f.
About the Authors
K. N. GrigorevaRussian Federation
Kristina N. Grigoreva – MD, Medical Resident, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
V. O. Bitsadze
Russian Federation
Victoria O. Bitsadze – MD, Dr Sci Med, Professor of RAS, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Scopus Author ID: 6506003478,
Researcher ID: F-8409-2017
J. Kh. Khizroeva
Russian Federation
Jamilya Kh. Khizroeva – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Scopus Author ID: 57194547147,
Researcher ID: F-8384-2017
E. V. Slukhanchuk
Russian Federation
Ekaterina V. Slukhanchuk – MD, PhD, Associate Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health, Sechenov University; Obstetrician-Gynecologist, Department of Abdominal Surgery and Oncology 2, Petrovsky National Research Centre of Surgery
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991,
2 Abrikosovskiy Lane, Moscow 119991
M. V. Tretyakova
Russian Federation
Maria V. Tretyakova – MD, PhD, Obstetrician-Gynecologist, Assistant, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
N. A. Makatsariya
Russian Federation
Nataliya A. Makatsariya – MD, PhD, Associate Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children's Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Researcher ID: F-8406-2017
J.-Ch. Gris
Russian Federation
Jean-Christophe Gris – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health, Sechenov University; University of Montpellier, Montpellier, France; Foreign Member of RAS
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991,
163 Rue Auguste Broussonnet, Montpellier 34090
Researcher ID: AAA-2923-2019
G. C. Di Renzo
Italy
Gian C. Di Renzo – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health, Sechenov University; Director of the Center for Prenatal and Reproductive Medicine, University of Perugia; Honorary Secretary General of the International Federation of Gynecology and Obstetrics (FIGO)
Umbria, Perugia, Piazza Italia
Scopus Author ID: 7103191096,
Researcher ID: P-3819-2017
V. I. Tsibizova
Russian Federation
Valentina I. Tsibizova – MD, PhD, Obstetrician-Gynecologist, Research Laboratory of Operative Gynecology, Institute of Perinatology and Pediatrics; Physician, Department of Functional and Ultrasound Diagnostics
2 Akkuratova Str., Saint Petersburg 197341
D. V. Blinov
Russian Federation
Dmitry V. Blinov – MD, PhD, MBA, Head of Medical and Scientific Affairs, Institute for Preventive and Social Medicine; Neurologist, Lapino Clinical Hospital, MD Medical Group
4–10 Sadovaya-Triumfalnaya Str., Moscow 127006,
1st Uspenskoe Highway, 111, Moscow Region, Odintsovo District, Lapino 143081
Scopus Author ID: 6701744871,
Researcher ID: E-8906- 2017,
RSCI: 9779-8290
A. D. Makatsariya
Russian Federation
Alexander D. Makatsariya – MD, Dr Sci Med, Academician of RAS, Professor, Head of the Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991
Researcher ID: M-5660-2016
Review
For citations:
Grigoreva K.N., Bitsadze V.O., Khizroeva J.Kh., Slukhanchuk E.V., Tretyakova M.V., Makatsariya N.A., Gris J., Di Renzo G.C., Tsibizova V.I., Blinov D.V., Makatsariya A.D. Preeclampsia and venous thromboembolism. Obstetrics, Gynecology and Reproduction. 2022;16(3):306-316. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.315

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































