Scroll to:
Nutrients and environmental toxicants: effect on placental function and fetal growth
https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.487
Abstract
Understanding the mechanisms by which environmental factors impact reproductive health is crucial for informing public health interventions and policy decisions. By elucidating the pathways through which environmental stressors exert their effects, we can develop targeted strategies to mitigate risks and promote reproductive well-being. In this lecture, we will delve into the latest research findings and emerging trends in the field of environmental reproductive health. By exploring the intricate interplay between environmental exposures and reproductive outcomes, we aim to broaden our understanding of this complex relationship and its implications for human health. Through collaborative efforts across disciplines, we can work towards safeguarding reproductive health for current and future generations.
For citations:
Di Renzo G.C. Nutrients and environmental toxicants: effect on placental function and fetal growth. Obstetrics, Gynecology and Reproduction. 2024;18(1):112-124. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.487
Introduction / Введение
In recent years, there has been a growing recognition of the profound influence that environmental factors exert on reproductive health. The term "environment" encompasses a wide array of elements, ranging from industrial and agricultural chemicals to physical agents like heat and radiation. It includes by-products of combustion and industrial processes, such as dioxins, as well as dietary factors like foods and nutrients. Additionally, prescription drugs, lifestyle choices, substance abuse, and social and economic factors all play significant roles in shaping the reproductive health landscape.
The intersection of environment and reproductive health has garnered increasing attention due to mounting evidence linking environmental stressors to adverse health outcomes. Particularly concerning are the strong associations between developmental exposures and reproductive health toxicity. Of paramount importance are the effects of prenatal and early-life exposures, which occur during critical windows of vulnerability.
During prenatal and early-life stages, the developing organism is highly susceptible to the influence of its surroundings. This vulnerability extends to gametes (oocytes and sperm), as well as the placenta, which serves as a vital interface between maternal and fetal environments. Importantly, research has highlighted the role of intergenerational and transgenerational transmission in perpetuating the effects of environmental exposures across multiple generations.
Environmental toxicants/stressors // Токсиканты/стрессоры окружающей среды
The prevalence of environmental toxicants and stressors poses a significant challenge to reproductive health worldwide. The scale of industrial chemical production and usage is staggering, with profound implications for human health. In the United States alone, the volume of domestically manufactured and imported industrial chemicals reached a staggering 9.5 trillion pounds in 2012. To put this into perspective that equates to over 30,000 pounds for every individual, underscoring the pervasive nature of chemical exposures in modern society.
Global chemical production has soared, increasing by a remarkable 23.5-fold between 1947 and 2007. In 2012 in USA 3.5 trillion Kilos of industrial chemicals domestically manufactured/imported (14 tons for each person). This exponential growth underscores the escalating demand for chemical compounds across various industries and sectors. Despite this surge in production, safety testing and regulatory oversight have often lagged behind, leaving many chemicals inadequately evaluated for their potential health risks.
Within this landscape, pesticides represent a significant category of concern, with approximately 900 active ingredients in use. Similarly, the presence of thousands of chemicals in food products (~ 3,000 active ingredients) further complicates the issue, raising questions about the long-term impacts of dietary exposures on reproductive outcomes. Moreover, the realm of pharmaceuticals and cosmetics introduces another layer of complexity, with an estimated 5,000 chemicals utilized in drug formulations and cosmetic products. While these substances serve vital therapeutic and cosmetic purposes, their potential effects on reproductive health warrant careful scrutiny. Future prognosis for chemical production growth is shown in Figure 1.
The prevalence of pesticide usage is notably high, with approximately 20 % of total pesticide use occurring in the United States alone. In regions such as China, where agricultural practices may be characterized by less intensive training among farmers, the reliance on pesticides remains significant. This is particularly pronounced in areas where valuable crops face heightened pressure from pests, such as Colombian coffee and Dutch tulips (Fig. 2).
Environmental chemicals exhibit transboundary movement facilitated by various means such as trade, food supply chains, atmospheric dispersion via wind currents, and hydrological transport through water systems. This global distribution leads to disparities and injustices in the dissemination of toxic substances across different regions of the world.
Routes of exposure to these chemicals encompass a diverse array of sources and compounds. Pesticides, heavy metals including mercury (Hg), and persistent organic pollutants such as dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyls (PCBs) are prominent contributors to environmental contamination. Additionally, compounds like phthalates, bisphenol A (BPA), electronic waste-derived brominated and chlorinated flame retardants, and household contaminants like polybrominated diphenyl ethers (PBDEs), phthalates, and formaldehyde further contribute to exposure risks.
Outdoor pollutants, including particulate matter, and lead, as well as hazardous substances from industrial emissions and chlorinated byproducts, pesticides, microorganisms, and a range of inorganic and organic chemicals, further compound the complexity of environmental exposure pathways. Moreover, the disposal of electronic waste, containing various hazardous components, adds to the environmental burden.
A significant proportion of chemicals undergo inadequate testing for potential adverse health effects prior to their introduction into the marketplace. Among the approximately 6,700 chemicals manufactured or imported into the United States, weighing over 25,000 pounds each, merely about 200 have undergone comprehensive health effect assessments. This leaves a vast majority of approximately 6,500 chemicals untested for their potential impacts on human health.
While pharmaceuticals undergo stringent safety evaluations before being approved for use, the same level of scrutiny is not uniformly applied to manufactured chemicals. The inception of regulatory oversight in the pharmaceutical industry, exemplified by the establishment of the Food and Drug Administration (FDA) in USA a century ago, marked a milestone in ensuring drug safety. However, it wasn't until such a landmark event as the incident with an incorrectly prepared sulfonamide antibiotic contained diethylene glycol as a solvent, which is toxic to humans and other mammals that caused mass poisoning in the United States in 1937, resulting in the death of more than 100 people, in 1938 the Food, Drug, and Cosmetic Act was enacted to regulate the production and circulation of pharmaceuticals, addressing issues of safety and efficacy. Subsequent regulation was implemented gradually, focusing initially on individual pharmaceuticals such as insulin and penicillin.
The USA Senate hearings conducted in June of 1960, under the chairmanship of Senator Estes Kefauver of the Subcommittee on Antitrust and Monopoly of the Committee on the Judiciary, focused on enhancing the drug provisions of the 1938 Act. These hearings led to the introduction of S.3815, a bill aimed at safeguarding public health through the implementation of specific manufacturing practices, expanding antibiotic certification requirements to encompass all antibiotics, and enacting other regulatory measures.
During the Kefauver hearings, FDA received a New Drug Application (NDA) for Kevadon, the brand of thalidomide intended for marketing in the United States by the William Merrell Company. Despite persistent pressure from the company, medical officer Frances Kelsey refused to approve the NDA due to insufficient safety data. By 1962, the devastating effects of thalidomide on newborns had become evident. Although Kevadon was never granted marketing approval, Merrell had already distributed over two million tablets for investigational use, a practice largely unchecked by existing laws and regulations. Subsequently, the FDA swiftly acted to retrieve the supply from physicians, pharmacists, and patients.
In recognition of her vigilance, Frances Kelsey received the President's Distinguished Federal Civilian Service Award in 1962, the highest civilian honor for government employees. The near catastrophe involving thalidomide spurred Senator Estes Kefauver to reintroduce his bill. On October 10, President Kennedy signed the Drug Amendments of 1962, also known as the Kefauver-Harris Amendments. These amendments mandated that drug manufacturers demonstrate to the FDA the safety and efficacy of their products before marke-
ting, expanded antibiotic certification requirements, and granted the FDA authority over prescription drug advertising. Additionally, the amendments transferred the review of antibiotic NDAs from the Division of Antibiotics to the FDA, further strengthening regulatory oversight in the pharmaceutical industry [1].
The link between environmental factors and non-communicable diseases / Связь между факторами окружающей среды и неинфекционными заболеваниями
The prevalence of non-communicable diseases (NCDs) has markedly increased, encompassing conditions such as obesity, diabetes, neurodevelopmental disorders, reproductive issues, respiratory problems, thyroid dysfunction, and various cancers. This rise coincides with a significant escalation in unregulated global chemical production, utilization, and disposal of hazardous chemicals. Emerging evidence suggests that environmental chemicals, including endocrine-disrupting compounds and air pollution play pivotal roles in the development of NCDs and the origins of health and disease (DOHaD).
It is now firmly established that dietary factors and nutrition exert profound influences on both chronic diseases and poverty-related health conditions. There has been a notable shift in the primary causes of premature mortality and disability worldwide, transitioning from infections and malnutrition, historically prevalent adversaries, to chronic, lifestyle-related, or NCDs such as heart disease, cancer, type 2 diabetes mellitus (DM-2), and obesity.
NCDs currently stand as the leading causes of death, disability, and disease globally, with the exception of sub-Saharan Africa. Projections from the World Health Organization (WHO) and the World Bank indicate that while major NCDs accounted for nearly 60 % of all deaths in 2001, this figure is expected to rise to 73 %.
The surge in disease incidence over the past four decades cannot be attributed to genetic alterations, as human genes remain relatively stable over this period. Rather, recent "epidemics" of conditions like diabetes, asthma, attention deficit hyperactivity disorder (ADHD), and obesity stem from environmental, dietary, and behavioral shifts. Without a comprehensive understanding of the role of the environment, the etiology of these diseases will remain elusive.
Several global dynamics are contributing to these changes, including the effects of globalization, shifts in dietary patterns towards ultra-processed foods, alterations in air quality both indoors and outdoors, changes in personal behaviors and habits, evolving diagnostic criteria and technological advancements, and heightened exposures to chemical substances.
Recent research underscores the significance of maternal nutrition as a conduit for chemical stressors that impact the health of women and their offspring, emphasizing the critical interplay between environmental exposures and maternal and child health outcomes [2, 3].
We are inundating our environment with untested and hazardous chemicals, and the repercussions on our reproductive health are deeply concerning. Healthcare providers specializing in reproductive health observe a rising incidence of health issues among their patients, highlighting the importance of mitigating exposure to toxic substances to alleviate burdens on women, children, and families globally [4].
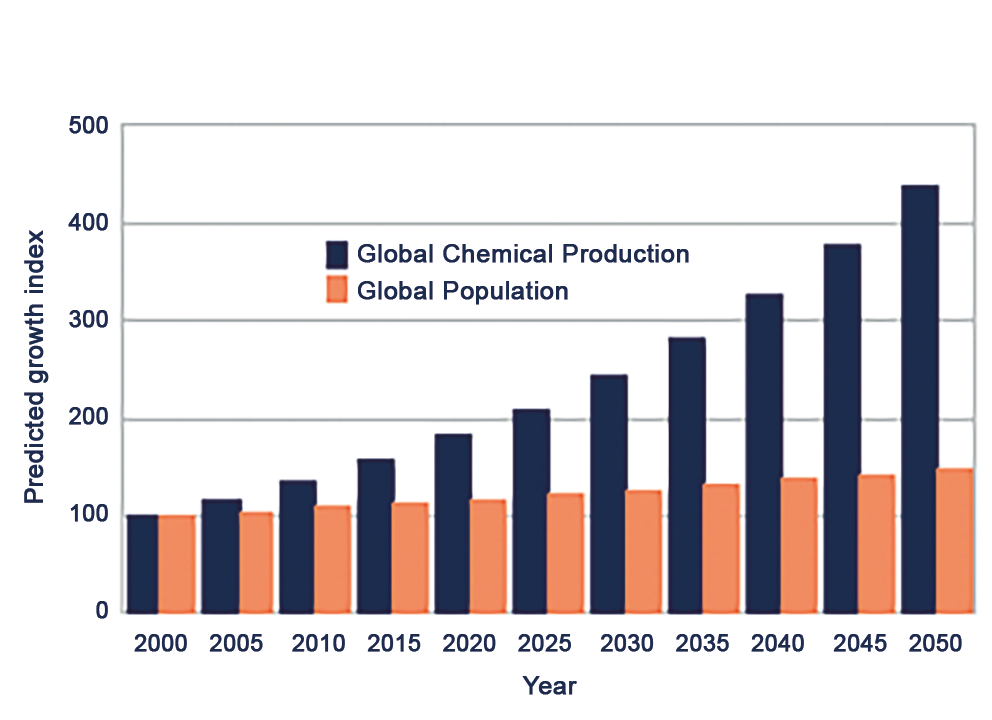
Figure 1. Growth in chemical production.
Рисунок 1. Рост химического производства.
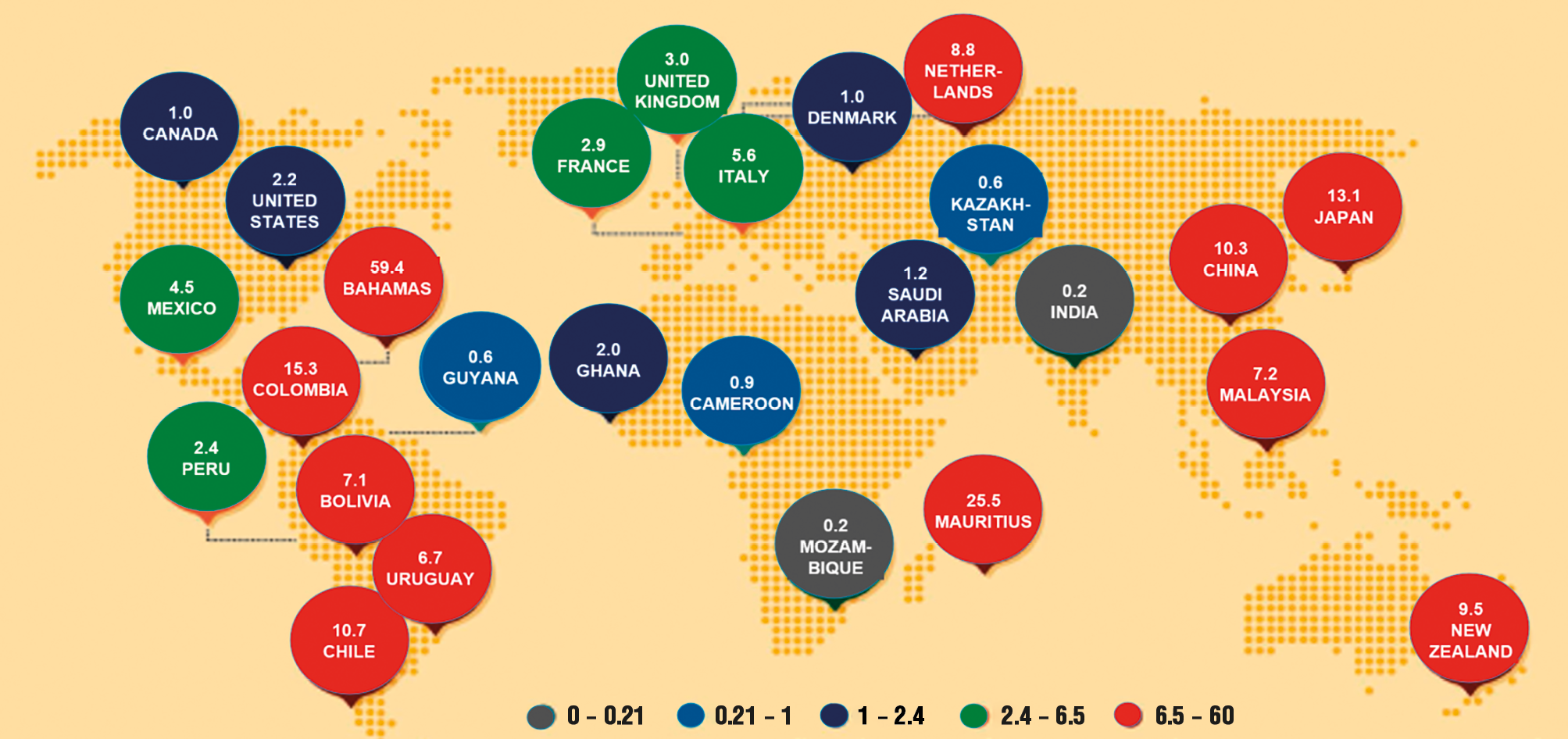
Figure 2. Pesticides applied kg/ha arable land, 2005-2009 (Science, 2013).
Рисунок 2. Применение пестицидов, кг/га пахотных земель, 2005–2009 гг. (Science, 2013).
Are we forgotting past evidence? / Неужели мы забываем прошлый опыт?
Throughout history, there have been significant instances of chemical exposures with enduring health ramifications. Examples include the widespread use of Diethylstilbestrol (DES) in the 1950s, methylmercury exposure in the 1960s, and the emergence of endocrine disruptors in the twenty-first century. These instances highlight the complex interplay between parental pressure and medical responsibility, as exemplified by the DES story.
DES, the first synthetic estrogen developed in the United States, was prescribed to nearly 5 million women between 1938 and 1970 in an attempt to prevent miscarriages. However, subsequent research revealed grave health risks associated with DES exposure, particularly for DES daughters. These individuals face heightened risks of vaginal and cervical dysplasia, potentially leading to cancer. Additionally, they are at increased risk of uterine anomalies, including infertility, ectopic pregnancy, late miscarriage, and premature labor. This historical example underscores the critical importance of vigilance in medical decision-making and the need for comprehensive evaluation of the risks and benefits associated with pharmaceutical interventions. The methylmercury exposure is another example of significant chemical exposure with lasting health consequences (Fig. 3).
Exposure to substances such as DES and methylmercury can have profound and lasting effects on developmental outcomes, particularly neurologic health. The toxicants ingestion or exposure during critical periods of fetal development can lead to a range of neurological changes and impairments. Individuals may experience ataxia, characterized by impaired coordination and muscle control, as well as seizures, which result from abnormal electrical activity in the brain. Additionally, the toxicants can contribute to sensory impairments, including hearing and vision loss, further compromising the individual's ability to navigate their environment effectively. In severe cases, the toxicants exposure may result in cerebral palsy, a neurological disorder characterized by impaired muscle tone and movement. Furthermore, developmental exposure to these toxicants can lead to language deficits and impairments in fine motor function, hindering the individual's ability to communicate and perform everyday tasks. Overall, the neurological consequences of toxicants exposure underscore the
critical importance of minimizing environmental exposures to protect the developing brain and promote optimal developmental outcomes [5].
The "Alphabet Soup" of Synthetic Chemicals encompasses a diverse array of compounds with varying degrees of toxicity and environmental persistence. Some of the key substances included in this category are: Polychlorinated Biphenyls (PCBs), Polybrominated Biphenyls (PBBs), Dioxin, Bisphenol A (BPA), Perfluorooctanoic Acid (PFOA), Phthalates, Pesticides (e.g., DDT), Pharmaceutical (e.g., DES), Soy (baby formula). Overall, the "Alphabet Soup" of Synthetic Chemicals represents a complex and interconnected web of environmental pollutants with the potential to impact human health and the environment. Vigilance in monitoring and regulating these substances is essential to minimize risks and protect public health [4].
Lead is a toxic heavy metal that has been found in certain cosmetic products, including lipstick. Lead may be present in lipstick as a contaminant from raw materials or as a by-product of manufacturing processes. While lead levels in lipstick are typically low, prolonged exposure to even small amounts of lead can pose health risks, particularly for pregnant women and children. Ingestion of lead from lipstick can occur through unintentional ingestion or absorption through the skin. Regulatory agencies, such as FDA, monitor lead levels in cosmetics and set maximum allowable concentrations (Fig. 4).
Endocrine disruptors (EDCs) are a diverse group of chemicals or mixtures of chemicals that can interfere with hormone action at various stages of development and throughout the life course. These substances have the ability to mimic, block, or interfere with hormone signaling pathways, leading to a range of adverse effects on human health. EDCs can exert their effects on multiple organ systems and physiological processes, with significant implications for male and female reproduction, breast development and cancer, prostate cancer, neuroendocrinology, thyroid function, metabolism, and obesity.
In male and female reproduction, EDCs can disrupt the delicate hormonal balance necessary for normal reproductive function. Exposure to EDCs has been linked to reduced fertility, impaired sperm quality, menstrual irregularities, and other reproductive disorders.
Breast development and cancer are also influenced by EDCs exposure, with certain chemicals capable of promoting abnormal growth and proliferation of breast tissue. EDCs may increase the risk of breast cancer by mimicking estrogen or disrupting hormonal signaling pathways involved in breast cell growth and differentiation.
Prostate cancer is another area of concern, as EDCs have been shown to disrupt hormonal regulation in the prostate gland, potentially increasing the risk of cancer development.
In neuroendocrinology, EDCs can interfere with the delicate balance of hormones involved in brain development and function, leading to neurobehavioral abnormalities and cognitive deficits.
Thyroid function is particularly vulnerable to disruption by EDCs, as these chemicals can interfere with thyroid hormone production, transport, and signaling. Thyroid disruption has widespread effects on metabolism, growth, and development.
Metabolism and obesity are also influenced by EDCs exposure, with certain chemicals capable of altering metabolic processes and promoting weight gain. EDCs may disrupt hormonal signaling pathways involved in appetite regulation, energy metabolism, and fat storage, contributing to the development of obesity and related metabolic disorders [6].
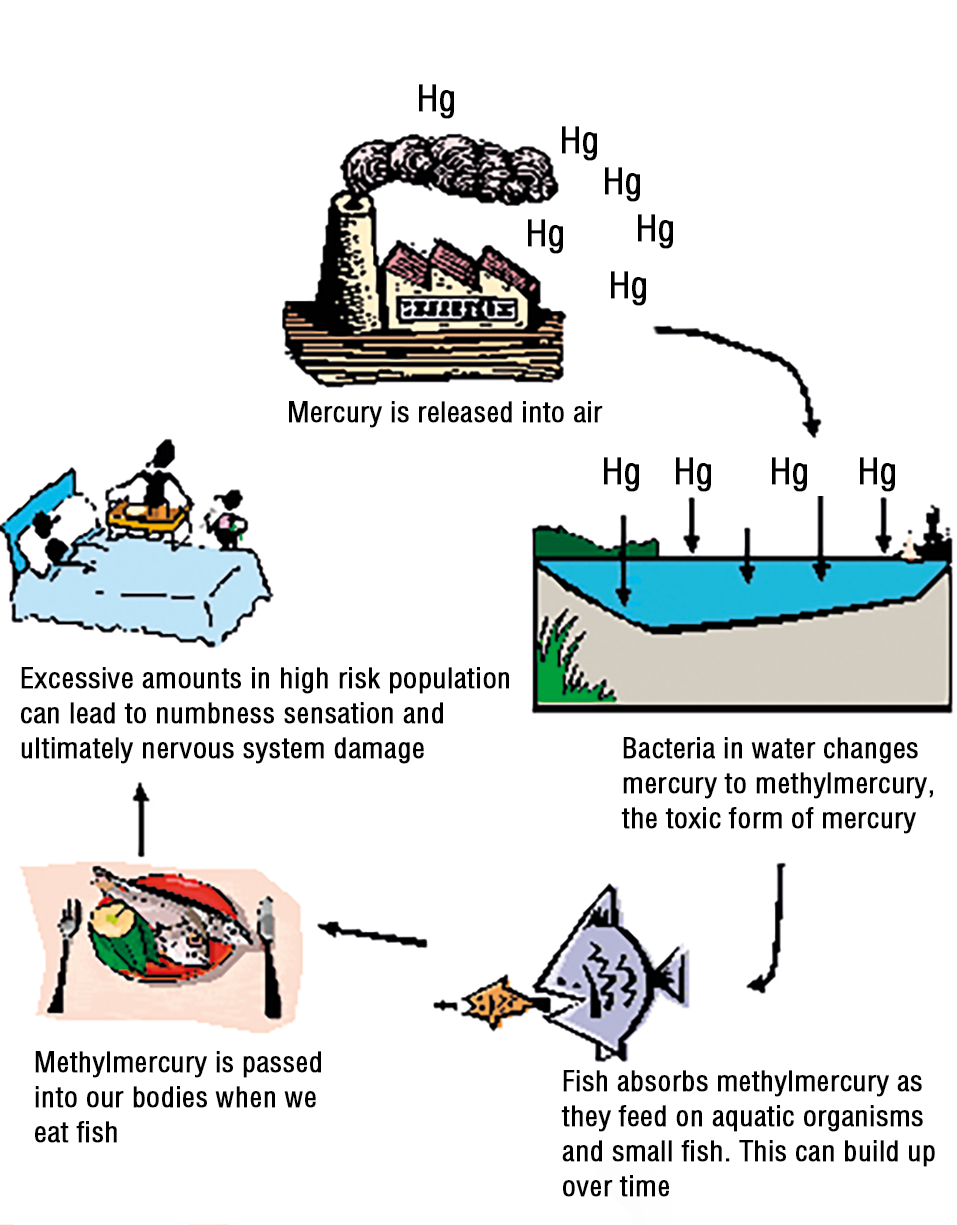
Figure 3. Methylmercury.
Рисунок 3. Метилртуть.

Figure 4. Lead in Lipstick.
Рисунок 4. Свинец в губной помаде.
Connections & new evidences / Новые данные
During the periconceptional period, optimal maternal nutrition plays a pivotal role in promoting healthy pregnancy outcomes and fetal development. A balanced diet during this critical window encompasses several key components: appropriate weight, body mass index (BMI) and weight gain; appropriate micronutrients supplementation; avoidance of alcohol, tobacco and other harmful/toxic substances; safe food handling (Fig. 5).
The concept of endocrine disruptors extends beyond their traditional impact on hormonal regulation to encompass emerging roles as obesogens and diabetogens. Pre- and perinatal exposures to these substances have been shown to disrupt the delicate balance of adipoge-
nesis and energy homeostasis, resulting in obesity in animal models. Epidemiological studies further corroborate these findings, linking exposure to specific endocrine disruptors such as BPA and phthalates with increased prevalence of obesity in human populations.
Animal models provide valuable insights into the mechanisms by which endocrine disruptors contribute to metabolic dysfunction and DM-2 development. Experimental evidence demonstrates that exposure to endocrine disruptors can adversely affect insulin production, secretion, and function, thereby disrupting glucose homeostasis and predisposing individuals to insulin resistance and DM-2. These findings underscore the intricate interplay between environmental exposures, endocrine disruption, and metabolic health, highlighting the need for continued research efforts to elucidate the underlying mechanisms and develop strategies for mitigating the adverse effects of endocrine disruptors on metabolic function and disease risk in human populations (Fig. 6).

Figure 5. Diets reduce the likelihood of adverse outcomes.
Рисунок 5. Диеты снижают вероятность неблагоприятных исходов.
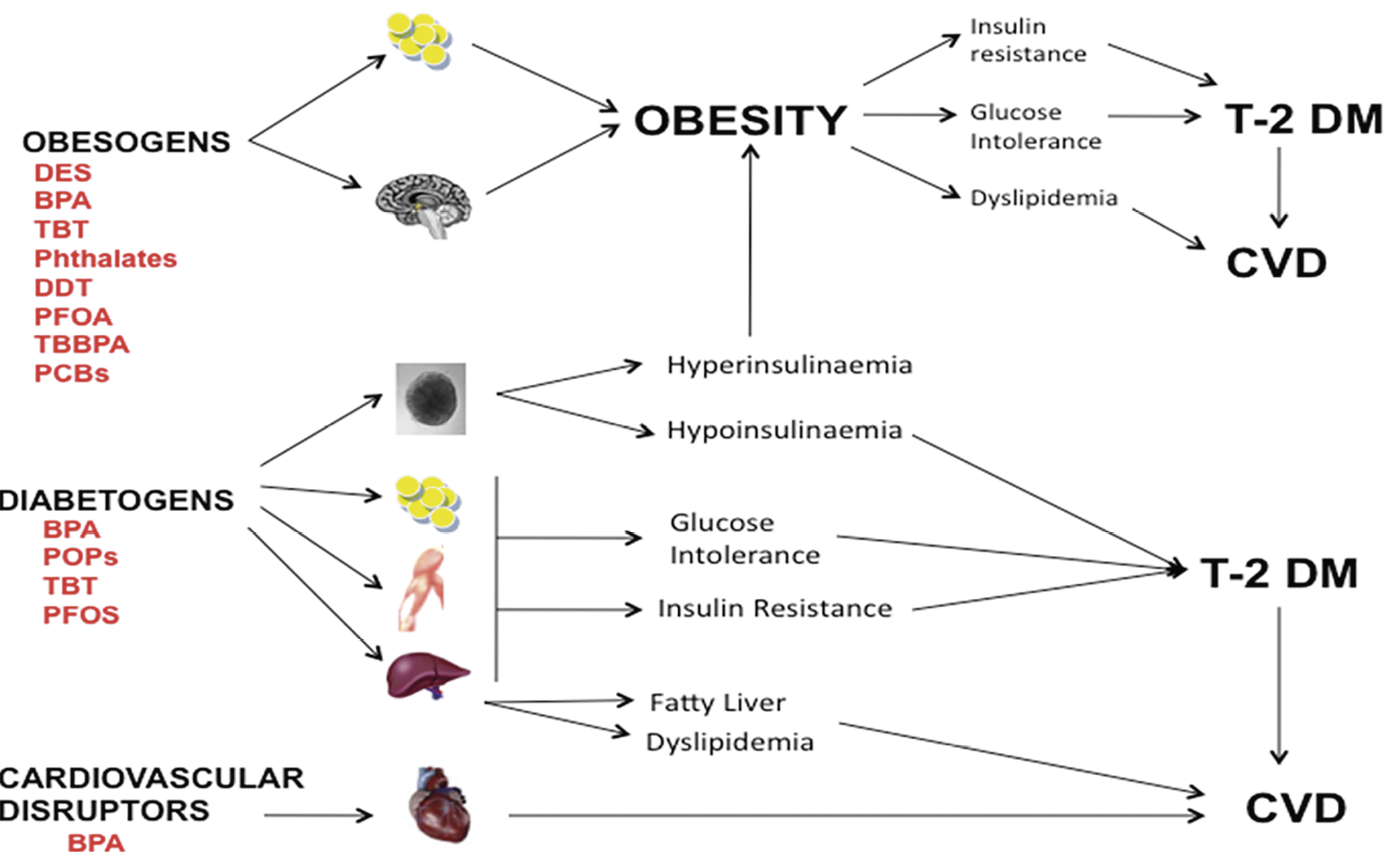
Figure 6. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus [7].
Рисунок 6. Эндокринные разрушители: потенциальный фактор риска развития гестационного сахарного диабета [7].
Research findings indicate a positive association
between maternal serum levels of Bisphenol A and the risk of miscarriage in women. Women with the highest BPA level in their serum exhibit a significantly elevated risk of miscarriage, encompassing both euploid and aneuploid pregnancies, compared to those with the lowest BPA level.
Moreover, a comprehensive review of nine studies, including seven cross-sectional investigations, one case-control study, and one pilot study, underscores the consistent impact of phthalates and organochlorines (OCs) on sperm quality abnormalities. Phthalates and OCs de-
monstrate a consistent and significant association with an increased risk of abnormal sperm quality, with odds ratios (OR) indicating elevated risks: phthalates (OR = 1.52; 95 % confidence interval [CI] = 1.09–1.95) and OCs (OR = 1.98; 95 % CI = 1.34–2.62) [8]. These findings emphasize the detrimental effects of environmental exposures to phthalates and OCs on male reproductive health and highlight the importance of further research to elucidate the underlying mechanisms and develop strategies for mitigating these risks (Fig. 7).
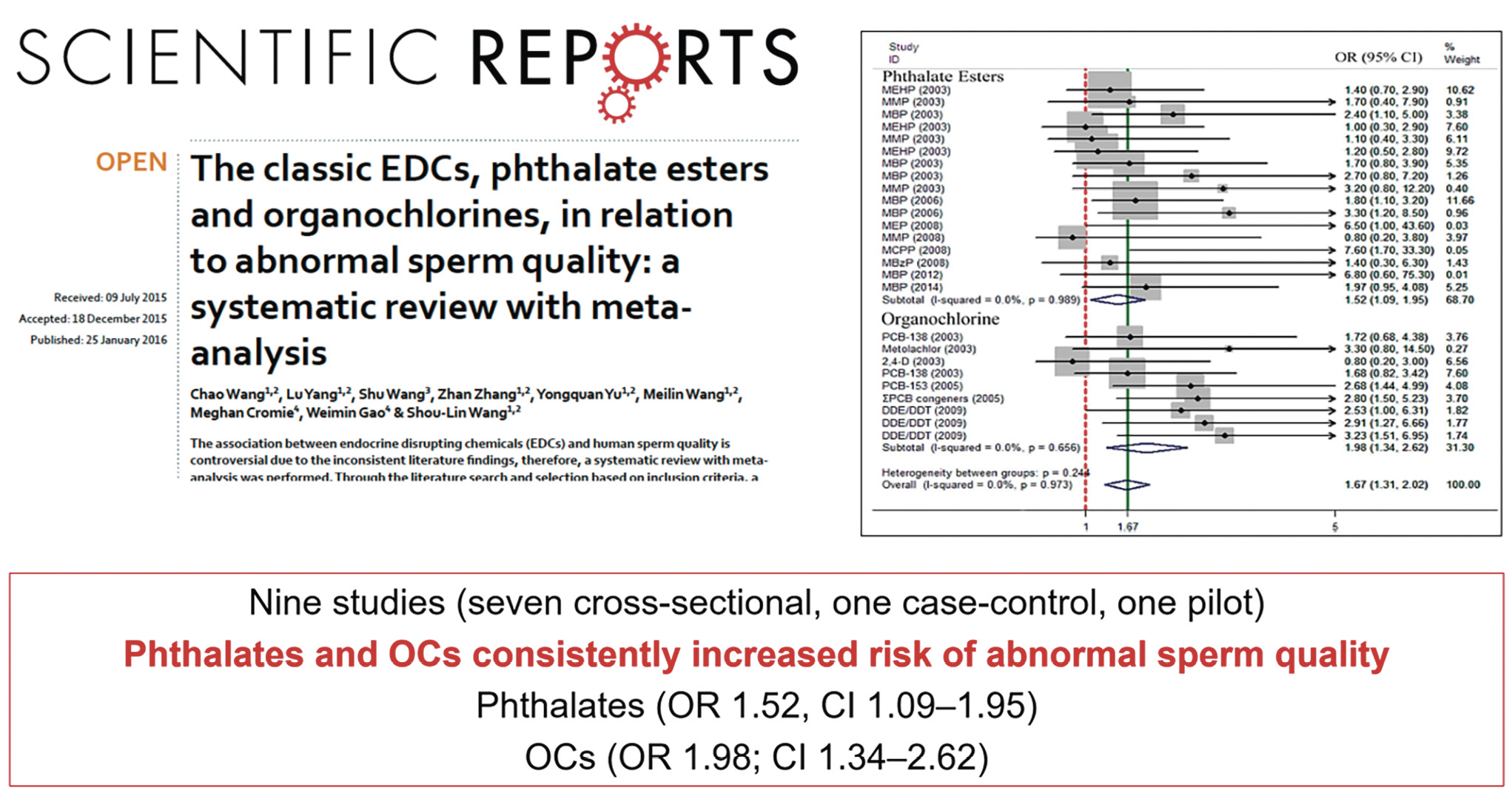
Figure 7. Endocrine disruptors and sperm quality [9].
Рисунок 7. Эндокринные разрушители и качество спермы [9].
A prospective observational nested case-control cohort study conducted between 2006 and 2008 investigated the relationship between maternal phthalate exposure and preterm birth (PTB). The study included 130 cases of PTB and 352 controls, with urine samples collected from participants at three time points during pregnancy and analyzed for levels of phthalate metabolites.
Upon analysis, adjusted models revealed that specific phthalate metabolites, including mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), and the sum of di(2-ethylhexyl) phthalate (ΣDEHP) metabolites, were associated with significantly increased odds of PTB. Furthermore, when spontaneous PTB was examined independently, additional phthalate metabolites, including MEHP, MECPP, ΣDEHP, MBP, and mono(3-carboxypropyl) phthalate, were also associated with significantly elevated odds of PTB [10]. These findings underscore the potential role of maternal phthalate exposure in the etiology of PTB and highlight the importance of further research to elucidate the mechanisms underlying this association. Additionally, efforts to mitigate maternal exposure to phthalates may have implications for reducing the incidence of PTB and improving maternal and neonatal health outcomes.
In the EARTH prospective cohort study spanning from 2007 to 2016, higher consumption of fruits and vegetables (FVs) with elevated pesticide residues was found to be associated with diminished likelihood of achieving pregnancy and live birth following assisted reproductive technology (ART). The study, comprising 325 women who completed diet assessment questionnaires and underwent a total of 541 ART cycles, meticulously categorized FVs into high or low pesticide residue groups utilizing a validated method based on surveillance data from the US Department of Agriculture.
The results revealed a compelling relationship between increased intake of high-pesticide residue FVs and reduced probability of clinical pregnancy and live birth: greater intake of high-pesticide residue FVs was associated with lower probability of clinical pregnancy and live birth. Compared with women in the lowest quartile of high-pesticide FV intake (< 1.0 servings/day), women in the highest quartile (2.3 servings/day) had 18 % (95 % CI = 5–30 %) lower probability of clinical pregnancy and 26 % (95 % CI = 13–37 %) lower probability of live birth [11].
Moreover, the pervasive exposure to polybrominated diphenyl ethers (PBDEs), commonly found in upholstered furniture, electronics, and the food supply, underscores the significance of environmental contaminants in influencing reproductive outcomes.
Furthermore, meta-analytical investigations in both humans and animals have identified the increased level of perfluorooctanoic acid (PFOA) as a significant factor associated with lower birth weight, highlighting the potential adverse effects of this ubiquitous environmental pollutant on fetal development.
Poly-Brominated Dyphenyl Ethers Ubiquitous Exposure (flame retardants): Upholstered Furniture, electronics, food supply.
What the Data Say about PFOA and BW? Meta-analysis in humans and animals finds PFOA associated with lower birth weight.
Also these is findings underscore the connection between adult diseases and pre-pregnancy and prenatal exposures to EDCs (Fig. 8) [12].
Environmental epigenetics, a burgeoning field of research, has garnered significant attention since the inception of the NIH Human Epigenome Project from 2008 to 2013. One intriguing aspect of this field is its revelation of intergenerational effects, where a woman's lifestyle choices can impact not only her immediate offspring but also her grandchildren. Epigenetics, the study of heritable changes in gene expression that occur without alterations to the DNA sequence, provides a mechanistic framework for understanding these phenomena. Processes such as gametogenesis and embryogenesis, as well as X-chromosome inactivation, play crucial roles in shaping the epigenome – the collection of DNA methylation marks, histone modifications, and small interfering RNAs (e.g., miRNAs) that regulate gene expression patterns. Notably, epigenetic modifications are influenced by a myriad of factors, including genetic variation and environmental exposures, underscoring the intricate interplay between nature and nurture in shaping individual health trajectories across generations.
Figure 8. Connection between adult diseases and pre-pregnancy and prenatal exposures to Endocrine Disruptors (EDCs) [12].
Рисунок 8. Связь между заболеваниями взрослых и воздействием эндокринных разрушителей (EDCs) до беременности и внутриутробно [12].
Placenta: a gatekeeper? / Плацента: посредник?
Transplacental passage is a multifaceted process influenced by various factors, with the placenta serving both permissive and protective roles against foreign substances, including antibiotics. The physicochemical properties of drugs, such as fat solubility, polarity, and molecular weight, critically influence their placental passage rates. Additionally, factors such as the surface area available for exchange, placental thickness, maternal-fetal blood flow, and fetal gender (sex) play pivotal roles in determining the placental passage of antibiotics.
Pregnant women encounter a myriad of environmental substances daily, and studies suggest that antenatal exposure to chemicals and pollutants may negatively impact fetal and neonatal growth, organ development, and long-term health outcomes, including obesity, metabo-
lic disorders, and preeclampsia. The placenta acts as a "gatekeeper" regulating the exchange between maternal and fetal environments, yet the mechanisms underlying transplacental passage of environmental contaminants remain incompletely understood.
Transplacental passage involves the transport of nutrients, elimination of metabolic waste products, and endocrine activity. Mechanisms of transport include passive diffusion, active transporters, and the expression of a wide range of enzymes. Metals and nanoparticles can cross the placenta, accumulate in placental tissue, and transfer to fetal blood and organs, impacting birth weight and neurodevelopment. The genetic factors modulating placental toxicokinetics have been postulated, but remain unclear.
The rapid development of nanotechnology has led to increased exposure to engineered nanoparticles with unique properties, raising concerns about potential health effects, especially during pregnancy. Exposure to air pollutants during pregnancy further heightens concerns about potential risks for nanoparticle exposure in utero [13].
Transplacental passage and/or placenta exposition to wide range of contaminants could led to several pathogenetic mechanism such as: disruption of oxidative balance; increased placental inflammation state; modifications in placental epigenome; alterations in transport systems.
SLC (solute carriers) & ABC (APT binding cassette): superfamilies of genes coding for molecular transporters found in the placenta. They are all gestational age dependent.
Transplacental passage of contaminants can disrupt oxidative balance, induce placental inflammation, alter the placental epigenome, and affect transport systems. Solute carriers (SLC) and ATP-binding cassette (ABC) superfamilies of genes code for molecular transporters found in the placenta, with expression levels varying with gestational age.
Pregnant women are often excluded from drug trials, leading to limited knowledge on correct dosages and usage of medications during pregnancy. This poses risks of underdosing or overdosing and potential harm to the fetus [14]. There is also the potential for pregnancy-specific drug interactions as drugs crossing the syncytiotrophoblast could saturate transporters; indeed, certain antibiotics and toxic chemicals have been shown to block transporters!
Sex differences in placental transporters are intricately linked to the sexually dimorphic endocrine environment of the fetus. ABCG2 expression, for example, has been reported to be altered by progesterone and estradiol exposure in the BeWo cell line. Similarly, recent evidence has suggested that the adverse pregnancy outcomes in polycystic ovary syndrome could be caused by hormonal influence on amino acid transport across the placenta due to much higher circulating levels of androgens. As the endocrine environment is established with respect to fetal sex from as early as 8 weeks of gestation, there may be sex-specific differential expression levels of transporters in both the fetus and placenta during pregnancy.
The genetics of the human placenta are pivotal in shaping its function and response to environmental exposures, with far-reaching implications for toxicokinetics. Genotypes play a crucial role in determining the placenta's ability to metabolize and transport substances, influencing the extent to which various compounds may reach the developing fetus. Moreover, chromosomal aberrations, including aneuploidies and sequence variations, can disrupt normal placental development and function, potentially impacting its ability to regulate fetal exposure to environmental toxins. Additionally, epigenetic mechanisms and genetic imprinting, further modulate placental gene expression patterns, influencing its responsiveness to environmental stimuli and altering toxicokinetic processes [15].
Actions to be taken / Действия, которые необходимо предпринять
Early intervention strategies are crucial in mitigating the adverse effects of toxic chemical exposure, emphasizing the importance of proactive measures over reactive responses (“better the evening before than the morning after”). Education and awareness initiatives as well as change risk factors play a pivotal role in equipping individuals with the knowledge and understanding needed to identify and avoid potential sources of toxic exposure. There are recommendations for preventing exposure to toxic chemicals (Fig. 9). Recommendation 1: advocate for policies to prevent exposure to toxic environmental chemicals. Recommendation 2: work to ensure a healthy food system for all. Recommendation 3: make environmental health part of health care. Recommendation 4: champion environmental justice.
But gaps and heterogeneity exist in awareness level and education. Data derived from a recent online survey in USA reported that: percentage of health professionals who reported talking with their patients about air pollution exposure were highest among respondents in pediatrics/ neonatology (56 %) and lowest among obstetrics/gynecologists (0 %) [16].
Might Green Chemistry Help? Green chemistry represents a paradigm shift in which hazardous chemicals, processes, and products are replaced with safer alternatives, exemplified by the transition from diethylstilbestrol (DES) to bisphenol A (BPA), and subsequently to bisphenol S (BPS) and bisphenol F (BPF). However, recent evidence suggests that these alternatives may still pose risks of hormone disruption [17, 18].
While individual actions can contribute to reducing exposures to harmful chemicals, substantial progress requires policy changes. For instance, the correlation between the decrease in lead content in gasoline and the corresponding decline in blood lead levels underscores the effectiveness of policy interventions in mitigating environmental exposures and protecting public health (Fig. 10).
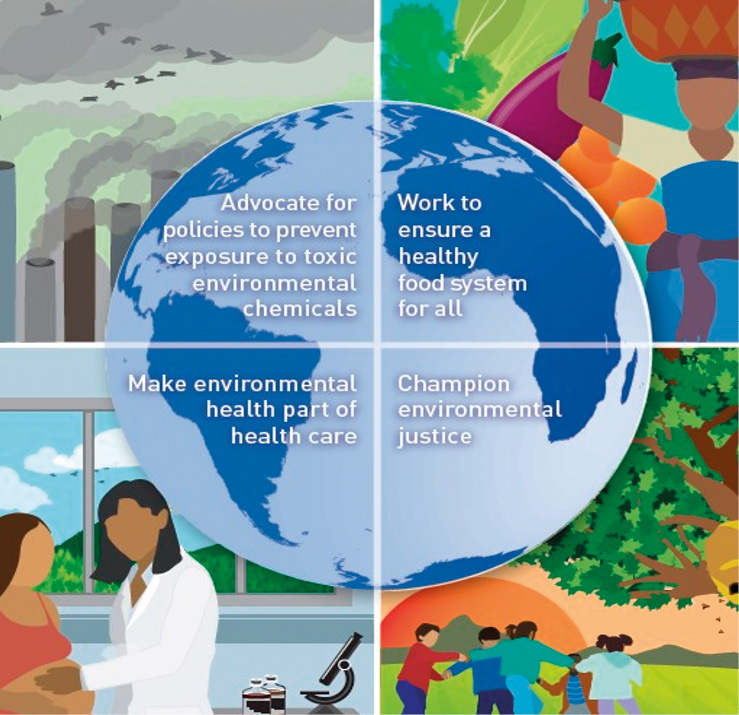
Figure 9. Recommendations for preventing exposure to toxic chemicals.
Рисунок 9. Рекомендации по предотвращению воздействия токсичных химических веществ.
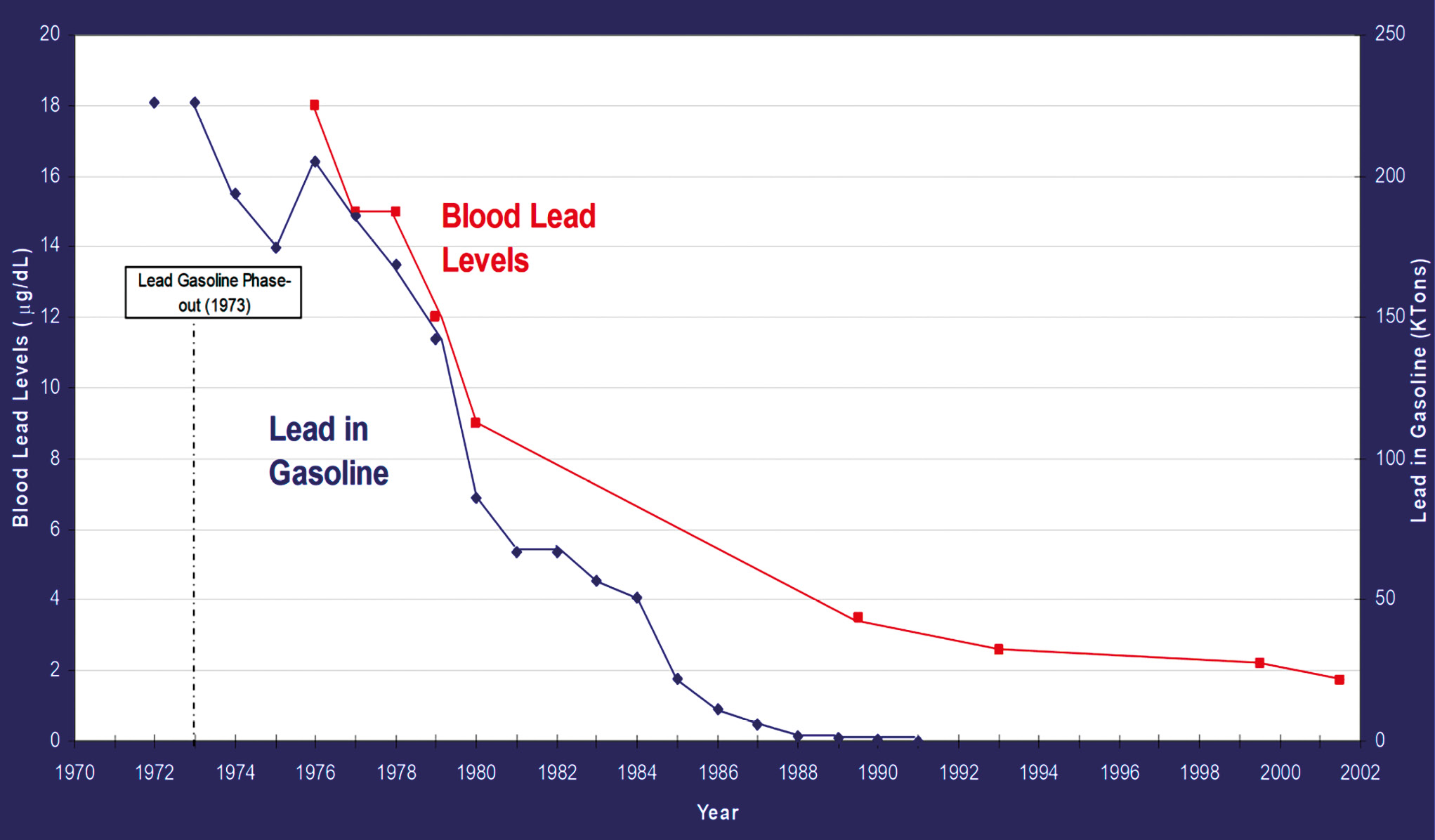
Figure 10. The relationship between a decrease in lead levels in gasoline and a decrease in blood lead levels.
Рисунок 10. Взаимосвязь между снижением уровня свинца в бензине и уменьшением уровня свинца в крови.
The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition provide comprehensive guidance for optimizing maternal and child health throughout the reproductive journey [19]:
- Provide appropriate nutrition and a healthy lifestyle before pregnancy
- Consider the weight and preconception body mass index as a modifiable risk factor
- Micronutrient deficiencies should be recognized and corrected through a varied diet, consumption of fortified foods, and supplementation if necessary
- Avoid smoking, alcohol intake, or use of recreational drugs before conception and during pregnancy
- During pregnancy every day do at least 30 minutes of moderate exercise
- During the later stages of pregnancy avoid extreme physical exercise and hard work
- During the II and III trimester most women should increase their dietary energy intake by approximately 340–450 kcal per day
- Reduce exposure to mercury, arsenic, lead and cadmium that could be ingested through food and water
- The period following birth must be used to improve the nutritional status of both mother and child
- Exclusive breastfeeding for the first 6 months of the infant's life
Here are recommendations for women seeking to minimize exposure to harmful substances and promote a healthy lifestyle (Fig. 11):
- Eat organic foods – really organic and fresh food
- Wash all fruits and vegetables and hands thoroughly (before eating)
- Minimize use of plastics, dry cleaning, canned goods, pesticides
- Avoid toxic “flame retardants”, read labels on furniture, clothing, baby products
- Avoid carbonless receipts
- Don’t use insecticides, bombs, or chalks
- No smoking, even 2nd hand
- Use alternative household cleaning products (soap, ammonia, baking soda)
- Phthalate-free make up, parabens, fragrances, triclosan, other personal care products
- Avoid hand sanitizers, digital paper receipt handling
- Avoid high PM air pollution if possible
- Avoid lead exposure (lead in unexpected places: ethnic products)
- Leave shoes at the door
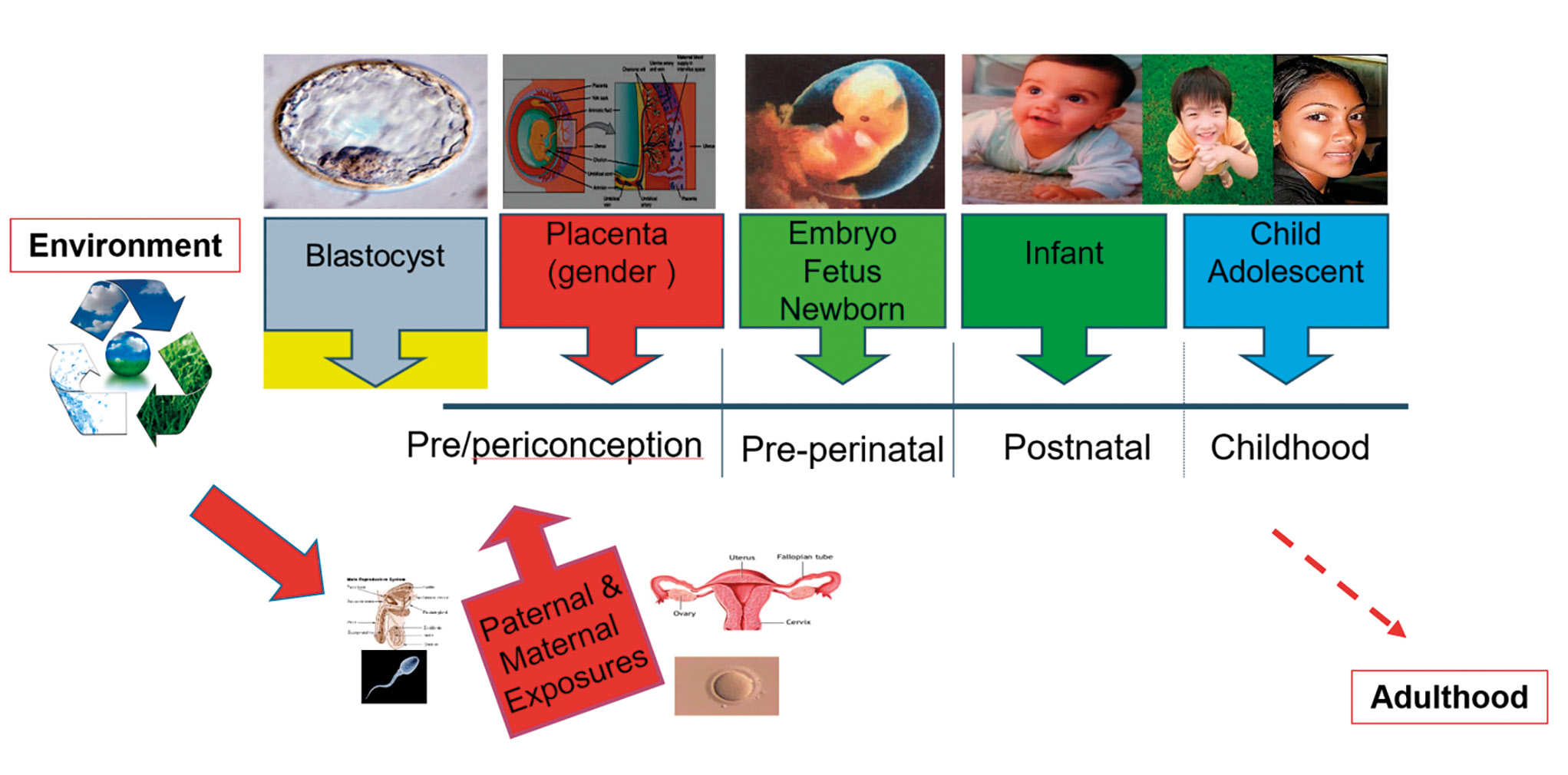
Figure 11. Periods of vulnerability to disruption by nutritional-environmental factors.
Рисунок 11. Периоды уязвимости к нарушениям в результате воздействия факторов питания и окружающей среды.
American marine biologist, writer and conservationist (1907–1964) Rachel Carson, who warned about the dangers of widespread use of pesticides, especially DDT, and its harmful effects on the environment and human health, wrote in her book "Silent Spring": “What we have to face is not an occasional dose of poison which has accidentally got into some article of food, but a persistent & continuous poisoning of the whole human environment” [20].
As we confront the myriad stressors threatening our ecosystems and health, it becomes increasingly imperative to comprehensively characterize these stressors and their impacts. The sheer volume of pollutants demands strategic interventions that prioritize critical windows of exposure and target specific tissues and functions, necessitating a deeper understanding of biological mechanisms, particularly the role of the placenta in mediating toxicity.
Institutional interventions, including governmental protections and policies, are essential to safeguarding public health and the environment against the relentless onslaught of toxins driven by economic interests. Collaboration among governments, international organizations like WHO and FIGO, non-state actors, industry stakeholders, and professional organizations is paramount in translating science knowledge into policy.
By uniting efforts across disciplines and sectors, we can bridge the gap between science and policy, ensuring that evidence-based interventions are implemented to mitigate the pervasive and insidious threat of environmental toxins. Only through concerted global action can we hope to address Carson's prescient warning of a "persistent and continuous poisoning of the whole human environment" and pave the way towards a healthier and more sustainable future for generations to come.
References
1. Pomper G.M. On ordinary heroes and American democracy. Routledge, 2016. 328 p.
2. Azad M.A.K., Liu G., Bin P. et al. Sulfur-containing amino acid supplementation to gilts from late pregnancy to lactation altered offspring’s intestinal microbiota and plasma metabolites. Appl Microbiol Biotechnol. 2020;104(3):1227–42. https://doi.org/10.1007/s00253-019-10302-6
3. Tang M., Xu C., Kun Chen K. et al. Hexachlorocyclohexane exposure alters the microbiome of colostrum in Chinese breastfeeding mothers. Environ Pollut. 2019;254(Pt A):112900. https://doi.org/10.1016/j.envpol.2019.07.068.
4. Di Renzo G.C., Conry J.A., Blake J. et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynecol Obstet. 2015;131(3):219–25. https://doi.org/10.1016/j.ijgo.2015.09.002
5. Gallo M.A., Doull J. History and scope of toxicology. In: Cassarett and Doull’s Toxicology: the basic science of poisons. Ed. Klassen C.D. 5th ed. New York: McGraw-Hill, 1996. 3–11.
6. Zoeller R.T., Brown T.R., Doan L.L. et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–110. https://doi.org/10.1210/en.2012-1422
7. Ehrlich S., Lambers D., Baccarelli A. et al. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am J Perinatol. 2016;33(13):1313–8. https://doi.org/10.1055/s-0036-1586500
8. Lathi R.B., Liebert C.A., Brookfield K.F. et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril. 2014;102(1):123–8. https://doi.org/10.1016/j.fertnstert.2014.03.024.
9. Wang C., Yang L., Wang S. et al. The classic EDCs, phthalate esters and organochlorines, in relation to abnormal sperm quality: a systematic review with meta-analysis. Sci Rep. 2016;6(1):19982. https://doi.org/10.1038/srep19982.
10. Ferguson K.K., McElrath T.F., Meeker J.D. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7. https://doi.org/10.1001/jamapediatrics.2013.3699.
11. Chiu Y.H., Williams P.L., Gillman M.W. et al. Association between pesticide residue intake from consumption of fruits and vegetables and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technology. JAMA Intern Med. 2018;178(1):17–26. https://doi.org/10.1001/jamainternmed.2017.5038.
12. Birnbaum L.S. When environmental chemicals act like uncontrolled medicine. Trends Endocrinol Metab. 2013;24(7):321–3. https://doi.org/10.1016/j.tem.2012.12.005
13. Bove H., Bongaerts E., Slenders E. et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10(1):3866. https://doi.org/10.1038/s41467-019-11654-3
14. Munić V., Kelnerić Z., Mikac L., Haber V.E. Differences in assessment of macrolide interaction with human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase activity and cellular accumulation assays. Eur J Pharm Sci. 2010;41(1):86–95. https://doi.org/10.1016/j. ejps.2010.05.016
15. Gundacker C., Neesen J., Straka E. et al. Genetics of the human placenta: implications for toxicokinetics. Arch Toxicol. 2016;90(11):2563–81. https://doi.org/10.1007/s00204-016-1816-6
16. Mirabelli M.C., Damon S.A., Beavers S.F., Sircar K.D. Patient–provider discussions about strategies to limit air pollution exposures. Am J Prev Med. 2018;55(2):e49–e52. https://doi.org/10.1016/j.amepre.2018.03.018
17. Kinch C.D., Ibhazehiebo K., Jeong J.-H. et al. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A. 2015;112(5):1475–80. https://doi.org/10.1073/pnas.1417731112
18. Eladak S., Grisin T., Moison D. et al. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103(1):11–21. https://doi.org/10.1016/j.fertnstert.2014.11.005.
19. Hanson M.A., Bardsley A., De-Regil L.M. et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: "Think Nutrition First". Int J Gynaecol Obstet. 2015;131 Suppl 4:S213–S253. https://doi.org/10.1016/S0020-7292(15)30034-5
20. Carson R. Silent spring. New Yorker, 1962.
About the Author
G. C. Di RenzoItaly
Gian C. Di Renzo – MD, Dr Sci Med, Professor, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health; Director; Honorary Secretary General of the International Federation of Gynecology and Obstetrics (FIGO), Scopus Author ID: 7103191096. Researcher ID: P-3819-2017.
2 bldg. 4, Bolshaya Pirogovskaya Str., Moscow 119991; Italy, Umbria, Perugia, Piazza Italia
Review
For citations:
Di Renzo G.C. Nutrients and environmental toxicants: effect on placental function and fetal growth. Obstetrics, Gynecology and Reproduction. 2024;18(1):112-124. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2024.487

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































